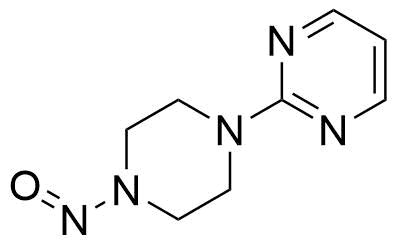
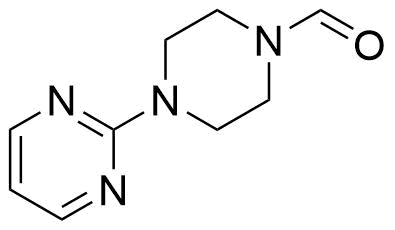
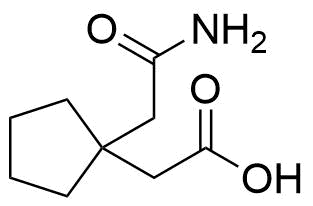
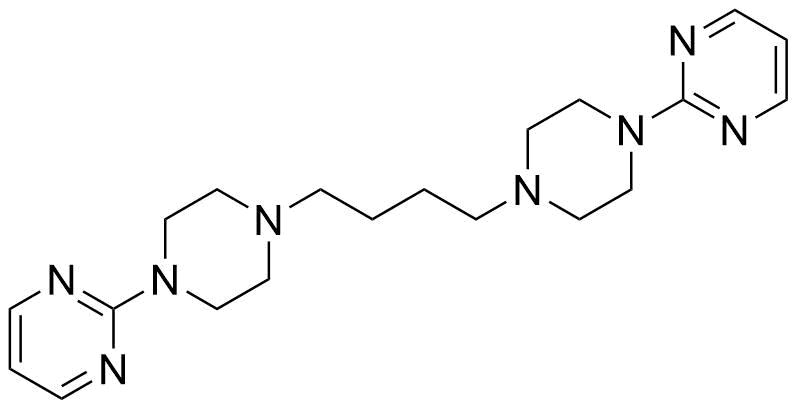
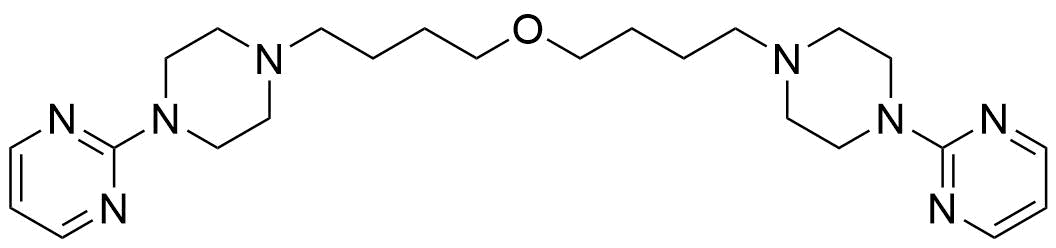
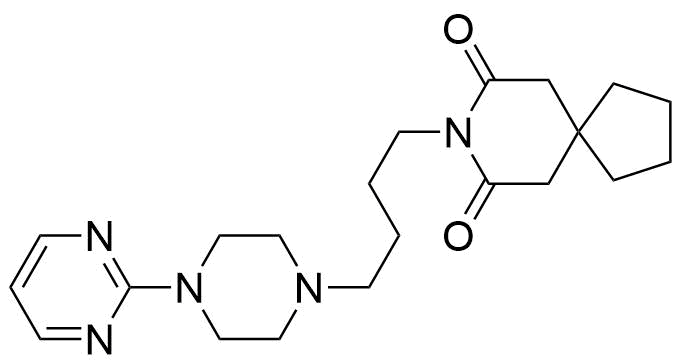
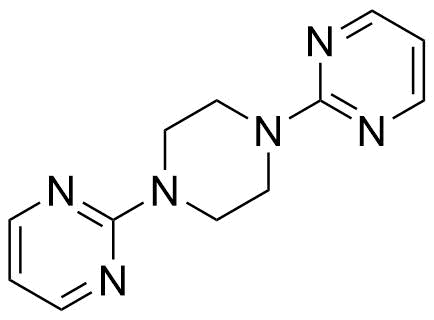
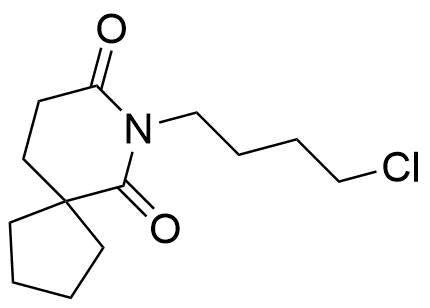
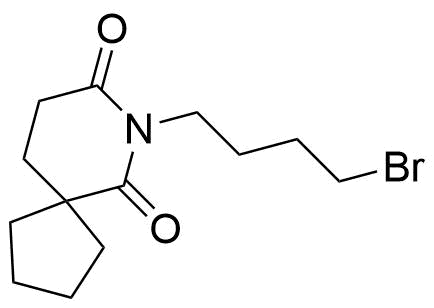
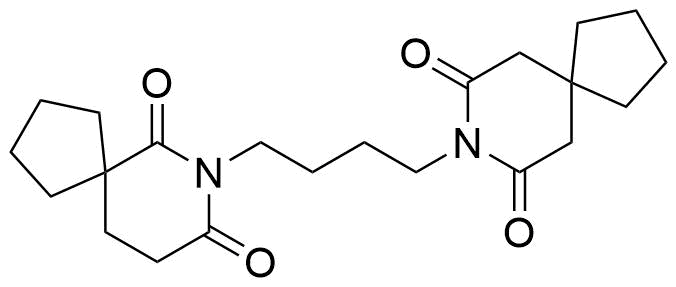
Buspirone Impurities
Buspirone is primarily used in the treatment of generalized anxiety disorder (GAD), offering a non-sedative alternative to benzodiazepines. It works by modulating serotonin and dopamine receptors, reducing anxiety without causing significant drowsiness or dependency. Typically taken in tablet form, it is a preferred option for long-term anxiety management, with fewer side effects compared to other anxiolytics. In pharmaceutical applications, ensuring the purity of Buspirone is crucial for its safety and effectiveness. Impurity profiling in Buspirone production involves identifying and quantifying potential by-products that may arise during synthesis, as these can impact drug quality. The detection of impurities is critical to meet regulatory standards, ensuring safe patient outcomes.
Inquiry