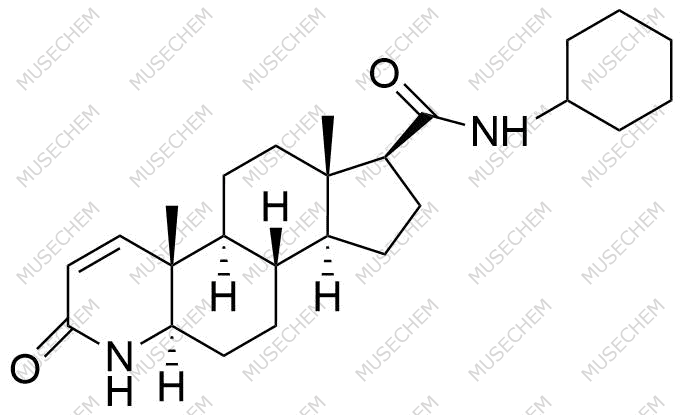
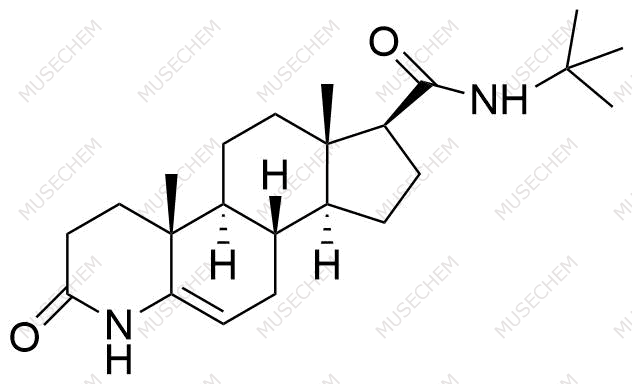
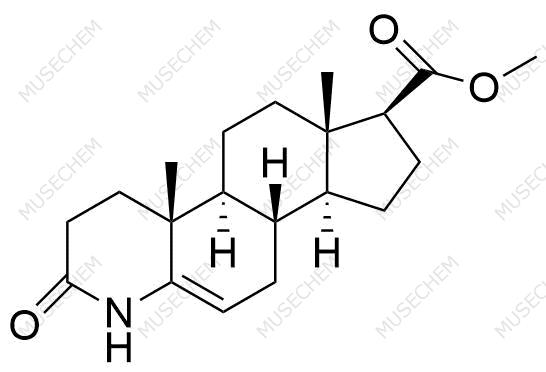
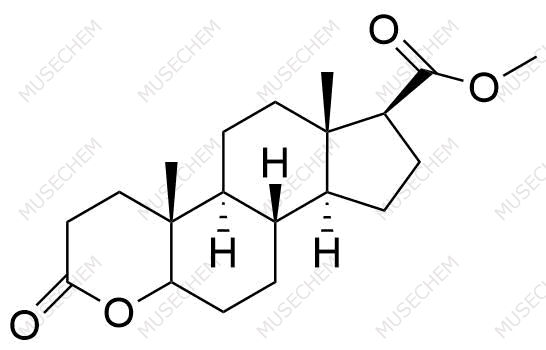
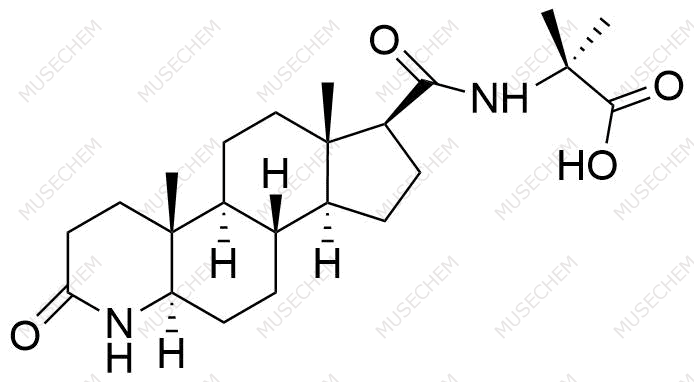
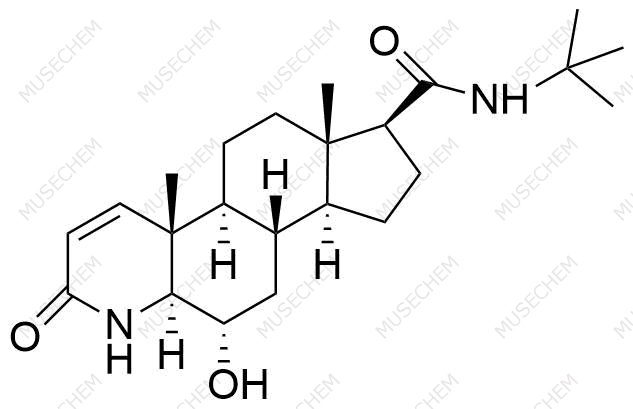
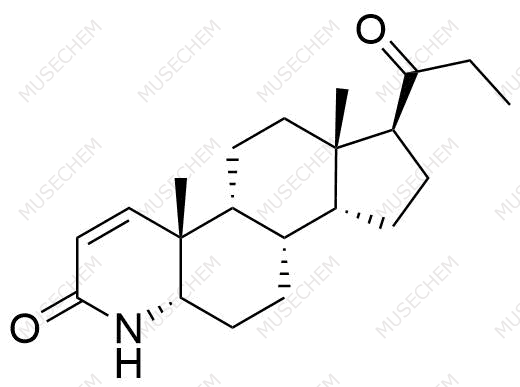
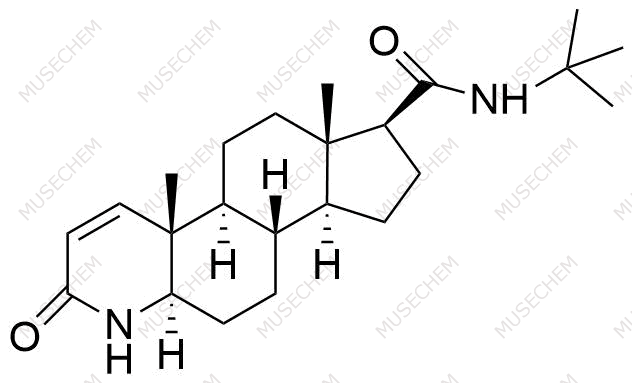
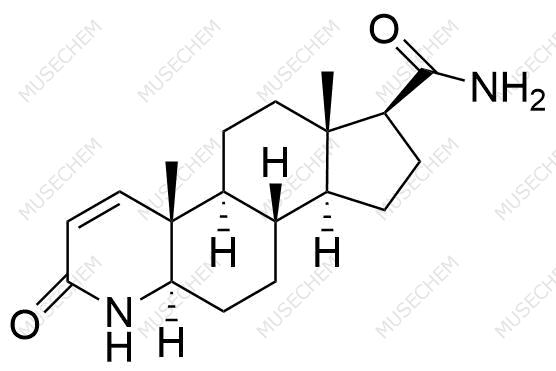
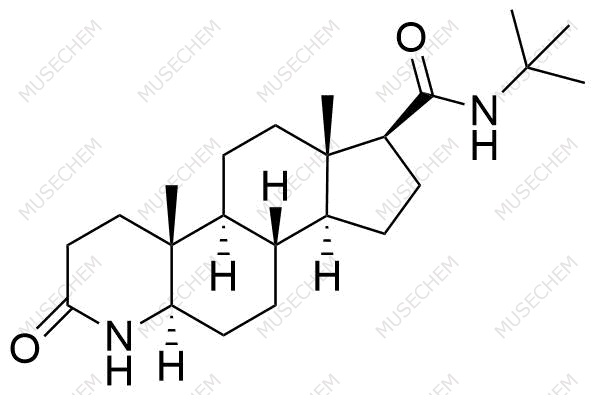
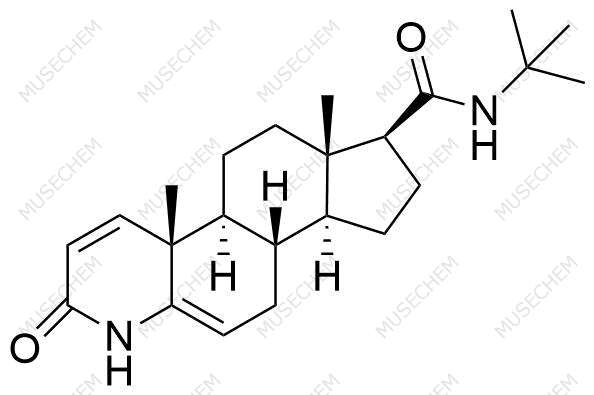
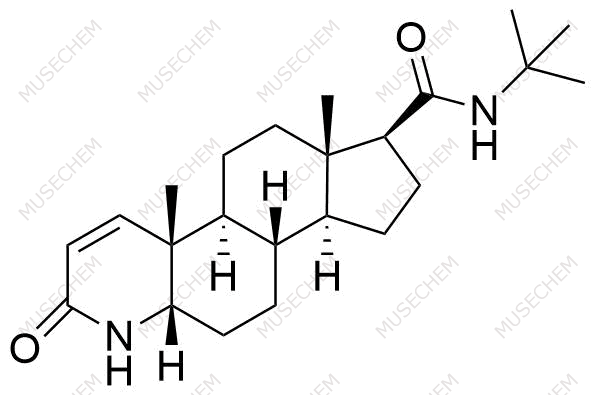
Finasteride Impurities
Finasteride is widely used in the treatment of benign prostatic hyperplasia (BPH) and male pattern baldness, targeting the 5-alpha-reductase enzyme to reduce dihydrotestosterone (DHT) levels. By decreasing DHT, Finasteride helps shrink the prostate in BPH and promotes hair regrowth in androgenetic alopecia. In pharmaceutical development, monitoring Finasteride-related impurities is critical for ensuring drug safety and efficacy. Common impurities may arise from synthesis, degradation, or storage, such as related compounds or residual solvents. Controlling these impurities is vital to maintaining the quality, stability, and regulatory compliance of the final product used in therapeutic applications.
Inquiry