A New Target for Immunotherapy—LAG-3
Abstract
Lymphocyte Activation Gene-3 (LAG-3) is a protein found on the surface of certain immune cells, including T cells and natural killer cells. LAG-3 plays a role in regulating immune responses by interacting with antigen-presenting cells, such as dendritic cells, and modulating T cell activation and function. LAG-3 has gained attention as a potential target for cancer immunotherapy, with several clinical trials underway testing LAG-3 inhibitors in combination with other immunotherapies. Additionally, LAG-3 has also been implicated in autoimmune disorders and may have potential as a target for treating these diseases.
Introduction to LAG-3: Structure, Function, and Expression
LAG-3 (lymphocyte activation gene 3) is a transmembrane protein expressed on the surface of a variety of immune cells, including T cells, B cells, and natural killer cells. The transmembrane protein LAG3 ( CD223 ) was first cloned in 1990 by Triebel and colleagues. LAG3 has a significant inhibitory effect on T cell activation. Studies have found that LAG3 is widely expressed in vivo and can be expressed by activated traditional and regulatory CD4+ and CD8+ T cells, natural killer (NK) cells and invariant NKT cells (invariant NKT Cells), as well as other immune and non-immune cells. The human LAG-3 gene is located on chromosome 12 and encodes a 498 amino acid protein.
In T cells, LAG3 expression can be induced by chronic inflammatory conditions, and LAG3 expression on exhausted tumor-infiltrating lymphocytes is associated with poor prognosis in several cancers. Like PD-1 and CTLA-4, LAG3 has also been identified as an immune checkpoint (immune brake) and is a popular therapeutic target for cancer immunotherapy.
The structure of LAG-3 consists of four extracellular domains, a transmembrane region and a short cytoplasmic tail. The extracellular domain contains an immunoglobulin-like domain and a unique “LAG-3-specific” domain that is involved in binding to MHC class II molecules on antigen-presenting cells.
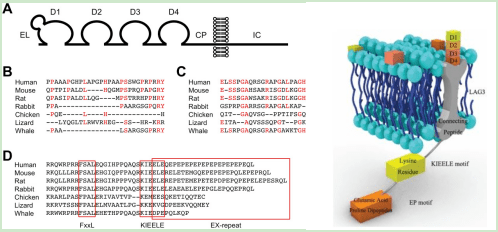
Figure 1 Structure of LAG-3
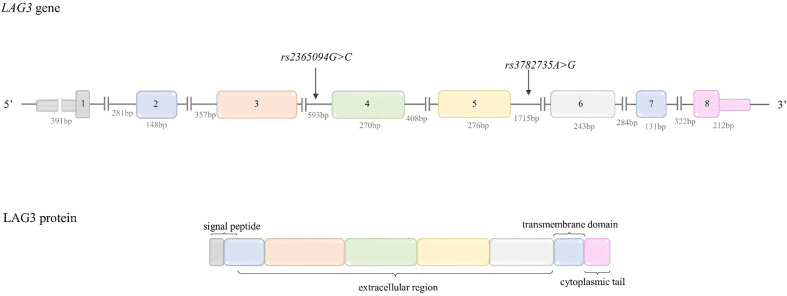
Figure 2 Structure of LAG-3 gene and protein
The function of LAG-3 is to regulate T cell activation and immune response. LAG-3 has been shown to act as a checkpoint molecule that inhibits T cell activation and proliferation when it binds to MHC class II molecules. This helps prevent an exaggerated immune response that can lead to autoimmunity and tissue damage.
The expression of LAG-3 is tightly regulated and depends on the activation state of T cells. LAG-3 is upregulated following T cell activation and is expressed at high levels on exhausted or dysfunctional T cells in chronic viral infection and cancer. This has led to the development of LAG-3 as a potential target for cancer immunotherapy.
In conclusion, LAG-3 is a transmembrane protein that regulates T cell activation and immune response. Its structure consists of four extracellular domains, a transmembrane region and a short cytoplasmic tail. LAG-3 is expressed on various immune cells and is upregulated following T cell activation.
LAG-3 signaling pathway
The molecular structure of LAG3 is comparable to that of CD4, but its affinity for binding to MHC II molecules is much stronger than that of CD4. Once bound to MHC II, LAG3 transmits negative regulatory signals through the TCR-CD3 complex. Experiments involving antibody cross-linking of human T cells have revealed that LAG3 associates with CD3 in TCR complexes, leading to reduced cytokine production and T cell proliferation.
Aside from its interaction with MHC II and CD3, LAG3 is also capable of binding to several other molecules such as FGL-1, α-syn, Gal-3, and LSECtin. In its role as an immune checkpoint, LAG3 can inhibit the activation of host cells and suppress the immune response.
LAG-3 expression is stimulated either by TCR activation or cytokines, specifically IL-12, IL-27, IL-15, IL-2, and IL-7. Once translated, LAG-3 is transported to the cell surface, where it exists as oligomers or monomers. Upon ligand binding, LAG-3 obstructs early TCR pathway events in a manner contingent on its cytoplasmic domain, effectively preventing the activation of transcription factors like NFAT. As a result, cytokine production and proliferation are reduced.
ADAM10/17 controls the surface manifestation of LAG-3, by cleaving the LAG-3 protein into two fragments: p54 and p16, which stay attached to the cell membrane. The p54 fragment with a molecular weight of 54 kDa contains D1, D2, and D3 domains, and is secreted as soluble LAG-2. On the other hand, the p16 fragment is the transmembrane-intracellular part with a molecular weight of 16 kDa. In addition, endocytosis can also regulate the surface expression of LAG-3, and ligand binding can expedite this process.
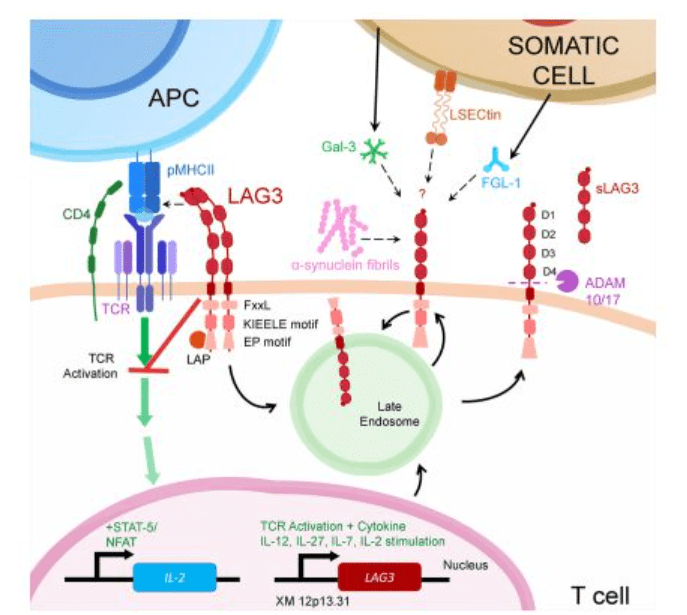
Figure 3 LAG-3 signal transduction diagram
Regulation of T Cell Activation by LAG-3
LAG-3 (Lymphocyte Activation Gene 3) is a checkpoint molecule that regulates T cell activation and immune responses. LAG-3 binds to MHC class II molecules on antigen-presenting cells (APCs) and modulates T cell signaling and activation in several ways.
Firstly, LAG-3 binding to MHC class II molecules can induce negative signaling pathways in T cells, leading to reduced T cell activation and proliferation. This helps to prevent excessive immune responses and tissue damage.
Secondly, LAG-3 can compete with CD4, another protein that binds to MHC class II molecules on APCs. Since CD4 is required for optimal T cell activation, LAG-3 binding can inhibit CD4-mediated T cell activation and proliferation.
Thirdly, LAG-3 can modulate the function of dendritic cells (DCs), a type of APC that plays a critical role in initiating T cell responses. LAG-3 binding to MHC class II molecules on DCs can impair their ability to stimulate T cells, leading to reduced T cell activation and proliferation.
Besides its role in regulating T cell activation and proliferation, LAG-3 is also involved in the differentiation and function of T cells. In particular, LAG-3 has been demonstrated to facilitate the differentiation of regulatory T cells (Tregs), a type of T cell that can suppress immune responses and sustain immune tolerance. This highlights the potential significance of LAG-3 in regulating the equilibrium between immune activation and tolerance.
Role of LAG-3 in Immune Tolerance and Autoimmunity
LAG-3 has a vital role in maintaining immune tolerance and preventing autoimmunity. It regulates T cell activation and differentiation, promotes the development of regulatory T cells (Tregs), and inhibits CD4-mediated T cell activation, leading to reduced immune responses and T cell proliferation. Dysregulation of LAG-3 has been linked to autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus. Therefore, understanding the function of LAG-3 in immune tolerance and autoimmunity is crucial in developing effective therapeutic approaches that target LAG-3 to treat these diseases.
LAG-3 as a Checkpoint Molecule in Cancer Immunotherapy
LAG-3 is a promising target for cancer immunotherapy. It is a checkpoint molecule that negatively regulates T cell activation and proliferation, and its overexpression on tumor-infiltrating lymphocytes (TILs) is associated with poor prognosis in various cancers.
In cancer immunotherapy, targeting LAG-3 can enhance the anti-tumor immune response by blocking its inhibitory signals on T cells. LAG-3 inhibition can also promote the differentiation and activity of effector T cells while reducing the activity of regulatory T cells, leading to a more robust and effective immune response against cancer.
Combining LAG-3 blockade with other checkpoint inhibitors such as anti-PD-1 or anti-CTLA-4 has shown promising results in preclinical studies and clinical trials. These combination therapies have demonstrated increased anti-tumor activity and improved patient outcomes in various cancers, including melanoma, non-small cell lung cancer, and renal cell carcinoma.
Furthermore, the safety and tolerability of LAG-3 targeting agents have been demonstrated in clinical trials, with manageable adverse effects. However, more research is needed to optimize the therapeutic efficacy of LAG-3 blockade and to identify biomarkers for patient selection and monitoring.
Clinical Trials Targeting LAG-3 in Cancer and Autoimmune Diseases
Lymphocyte activation gene 3 (LAG-3) is an immune checkpoint receptor that plays a crucial role in regulating immune responses. Recent research has focused on the development of clinical trials targeting LAG-3 in cancer and autoimmune diseases.
In cancer, LAG-3 is highly expressed on T cells that are exhausted, and it promotes tumor immune evasion. Therefore, LAG-3 blockade has emerged as a promising strategy for cancer immunotherapy. Several clinical trials are evaluating LAG-3-targeted therapies alone or in combination with other immune checkpoint inhibitors or chemotherapy in various cancer types, including melanoma, non-small cell lung cancer, and breast cancer.
LAG-3 is believed to contribute to immune dysregulation in autoimmune diseases. Ongoing clinical trials are investigating LAG-3-targeted therapies in conditions like rheumatoid arthritis and multiple sclerosis, with a particular emphasis on assessing safety, effectiveness, and pharmacokinetics.
Overall, targeting LAG-3 has the potential to improve the outcomes of cancer and autoimmune disease treatments by enhancing the immune response and reducing immune dysfunction.
Potential Mechanisms of Resistance to LAG-3 Blockade
LAG-3 (lymphocyte-activation gene 3) is an immune checkpoint receptor that plays a critical role in the regulation of T-cell function. The blockade of LAG-3 has shown promise as an immunotherapeutic strategy for the treatment of cancer. However, as with other immunotherapies, resistance to LAG-3 blockade can develop over time, leading to treatment failure. Here are some potential mechanisms of resistance to LAG-3 blockade:
Upregulation of other immune checkpoint receptors: Cancer cells can upregulate other immune checkpoint receptors such as PD-1, CTLA-4, or TIM-3, which can compensate for the loss of LAG-3 signaling, leading to immune escape.
Alterations in antigen presentation: LAG-3 blockade requires the presentation of tumor antigens to T-cells, but cancer cells can downregulate antigen presentation, limiting the effectiveness of LAG-3 blockade.
Immunosuppressive cell populations: Tumor-infiltrating myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (Tregs) can inhibit T-cell activation and function, leading to resistance to LAG-3 blockade.
Genetic mutations: Mutations in tumor cells can lead to the loss of LAG-3 expression or changes in downstream signaling pathways, leading to resistance to LAG-3 blockade.
Tumor heterogeneity: Tumor heterogeneity can lead to the emergence of LAG-3-negative tumor cell clones that are resistant to LAG-3 blockade, leading to treatment failure.
Understanding the mechanisms of resistance to LAG-3 blockade can guide the development of combination therapies to overcome resistance and improve the efficacy of LAG-3 blockade.
Research pattern
According to public data, there are currently more than 70 LAG3 drugs under research worldwide, most of which are in preclinical stages. Judging from this data, it is expected that the number of LAG3 drugs entering clinical practice will continue to grow in the next few years.
Cancer types involved include solid tumors, breast cancer, ovarian cancer, melanoma, kidney cancer, colorectal cancer, liver cancer, pancreatic cancer, and blood cancer.
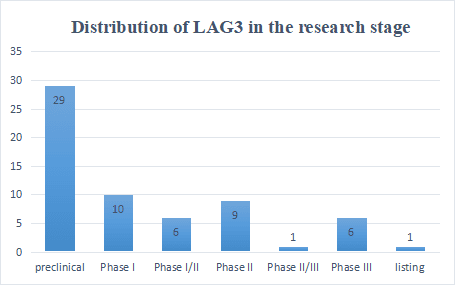
Most of them are monoclonal antibodies, and most of the double-antibodies are the combination of LAG3 and PD(L)1, TIGIT and CTLA-4. In addition, the combination of LAG3 monoclonal antibody, PD(L)1 monoclonal antibody and TGIIT monoclonal antibody is currently tried by most companies. direction.
This is the first immunotherapy targeting a new immune checkpoint protein approved by the FDA in the past 10 years. Compared with other immune checkpoint combination therapy, the co-expression of PD-1 and LAG-3 on activated T cells contributes to the rapid transport of their signals and chemicals to the immune synapse, thus showing a positive effect on T cell signaling. lead to a stronger synergistic inhibitory effect.
The combined use of PD-1 and LAG-3 can also avoid the compensatory induction of LAG-3 and CTLA-4 and other checkpoint pathways caused by a single checkpoint blockade of PD-1, thereby avoiding the formation of feedback loops caused by Decreased efficacy of monotherapy.
Future Directions for LAG-3 Research and Therapeutic Applications
LAG-3 (Lymphocyte-activation gene 3) has emerged as a promising immune checkpoint target for cancer and autoimmune diseases, but further research is needed to advance its therapeutic applications. Future directions include identifying patient populations that would benefit from LAG-3-targeted therapies and understanding the interplay between LAG-3 and other immune checkpoints. Developing more potent and specific LAG-3 inhibitors, such as small molecule inhibitors or bispecific antibodies, could enhance efficacy and reduce toxicity. Investigating the potential of LAG-3-targeted therapies in combination with other immunotherapies may provide further benefits. Understanding the mechanisms of LAG-3-mediated immune regulation and its role in T-cell exhaustion could lead to the development of novel therapeutic strategies.
Exploring the potential of LAG-3-targeted therapies in different cancer types and assessing their safety and efficacy in early-phase clinical trials should be a priority. Identifying biomarkers that can predict the response to LAG-3-targeted therapies will facilitate patient selection and improve clinical outcomes. Investigating the potential of LAG-3 as a target for immunotherapy in non-cancer diseases, such as infectious diseases and autoimmune disorders, may offer new therapeutic avenues. Continued research into LAG-3 and its potential as a therapeutic target is likely to lead to significant advances in cancer and immune-related diseases.
References:
- Maruhashi T, Sugiura D, Okazaki IM, Okazaki T. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020 Sep;8(2):e001014. doi: 10.1136/jitc-2020-001014. PMID: 32929051; PMCID: PMC7488795.
- Wagner M, Jasek M, Karabon L. Immune Checkpoint Molecules-Inherited Variations as Markers for Cancer Risk. Front Immunol. 2021 Jan 14;11:606721. doi: 10.3389/fimmu.2020.606721. PMID: 33519815; PMCID: PMC7840570.
- Sauer N, Szlasa W, Jonderko L, Oślizło M, Kunachowicz D, Kulbacka J, Karłowicz-Bodalska K. LAG-3 as a Potent Target for Novel Anticancer Therapies of a Wide Range of Tumors. Int J Mol Sci. 2022 Sep 1;23(17):9958. doi: 10.3390/ijms23179958. PMID: 36077354; PMCID: PMC9456311.
- Lythgoe MP, Liu DSK, Annels NE, Krell J, Frampton AE. Gene of the month: lymphocyte-activation gene 3 (LAG-3). J Clin Pathol. 2021 Sep;74(9):543-547. doi: 10.1136/jclinpath-2021-207517. Epub 2021 Jun 28. PMID: 34183437.
- Huo JL, Wang YT, Fu WJ, Lu N, Liu ZS. The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front Immunol. 2022 Jul 26;13:956090. doi: 10.3389/fimmu.2022.956090. PMID: 35958563; PMCID: PMC9361790.
- Perez-Santos M, Anaya-Ruiz M, Cebada J, Bandala C, Landeta G, Martínez-Morales P, Villa-Ruano N. LAG-3 antagonists by cancer treatment: a patent review. Expert Opin Ther Pat. 2019 Aug;29(8):643-651. doi: 10.1080/13543776.2019.1642873. Epub 2019 Jul 18. PMID: 31291131.
- Burnell SEA, Capitani L, MacLachlan BJ, Mason GH, Gallimore AM, Godkin A. Seven mysteries of LAG-3: a multi-faceted immune receptor of increasing complexity. Immunother Adv. 2021 Dec 20;2(1):ltab025. doi: 10.1093/immadv/ltab025. PMID: 35265944; PMCID: PMC8895726.
- Rodríguez-Guilarte L, Ramírez MA, Andrade CA, Kalergis AM. LAG-3 Contribution to T Cell Downmodulation during Acute Respiratory Viral Infections. Viruses. 2023 Jan 3;15(1):147. doi: 10.3390/v15010147. PMID: 36680187; PMCID: PMC9865459.
- Shan C, Li X, Zhang J. Progress of immune checkpoint LAG-3 in immunotherapy. Oncol Lett. 2020 Nov;20(5):207. doi: 10.3892/ol.2020.12070. Epub 2020 Sep 8. PMID: 32963613; PMCID: PMC7491111.




