ADC Detailed Explanation Series: Target Selection in Cancer Therapy
Abstract
The article provides a comprehensive overview of antibody-drug conjugates (ADCs) and their targets in cancer therapy. It explores the rationale behind ADC development, emphasizing the importance of target selection, linker stability, and payload potency. The chapter discusses various targets used in ADC therapy, such as HER2, CD30, and CD33, highlighting their role in tumor cell targeting. It also covers the mechanism of action of ADCs, including target binding, internalization, and payload release.
What is ADC in pharmaceutical sector?
ADC (Antibody–Drug Conjugates), the full name of “antibody-drug conjugates”, is a type of targeted drug composed of antibodies, linkers, and cytotoxic drugs. It uses monoclonal antibodies as carriers to efficiently transport cytotoxic drugs in a targeted manner. into target tumor cells, combining the powerful killing effect of traditional chemotherapy and the tumor targeting of antibody drugs.
The ideal ADC drug remains stable in the blood circulation, accurately reaches the therapeutic target, and ultimately releases the cytotoxic payload near the target (such as cancer cells). For ADC drugs to achieve ideal efficacy and safety, five core elements need to be comprehensively considered when constructing ADC: target, antibody, linker, toxin, and conjugation method.
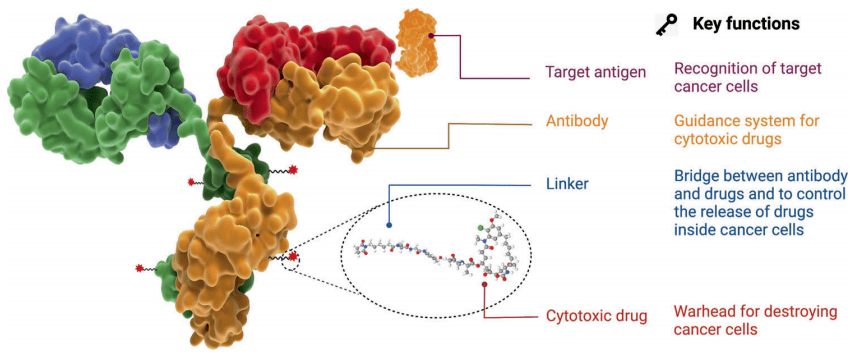
Figure 1. Structure and properties of ADC drugs
The mechanism of action of ADCs starts with their binding to target cells, guided by the presence of specific antigens on tumor cells. Once bound, ADCs are internalized through endocytosis and transported within the tumor cell. Ultimately, the ADC undergoes degradation, releasing the cytotoxic payload to induce cell death. Selecting the appropriate target antigen is critical for ADC efficacy, as it determines the specificity of the therapy for tumor cells. By targeting antigens expressed predominantly on cancer cells, ADCs can deliver cytotoxic drugs specifically to tumor cells, maximizing therapeutic benefit while minimizing off-target effects.
Target properties: an ideal target should have the following characteristics:
(1) Strong tissue specificity
To minimize off-target toxicity, an ideal ADC target should be highly specific to tumor tissues, with minimal or no expression in normal cells. For instance, HER2 is overexpressed about 100 times more in certain tumors than in normal cells. This specificity helps ensure that the ADC predominantly targets cancer cells, enhancing its therapeutic efficacy while reducing the risk of harming healthy tissues. Such precise targeting is crucial for improving treatment outcomes and minimizing side effects associated with traditional chemotherapy, highlighting the importance of selecting antigens that exhibit strong tissue specificity for ADC development.
(2) High antigen stability
To maintain effective tumor targeting and avoid safety concerns, the ideal ADC target antigen should be stable and located on the cell surface or extracellularly, rather than intracellularly. Intracellular antigens are less accessible to ADCs and may lead to reduced efficacy. Additionally, the target antigen should not be secreted to prevent detection by the circulatory system, which could result in off-target effects. A stable, surface or extracellular target antigen enhances the specificity of ADCs for cancer cells, improving their ability to deliver cytotoxic payloads precisely to tumor cells while minimizing systemic toxicity.
(3) Efficient internalization of antigen
Efficient internalization of the target antigen is crucial for ADC efficacy. Upon binding to the corresponding antibody, the ideal target antigen should undergo internalization, allowing the ADC to enter cancer cells. This internalization process enables the ADC to navigate through appropriate intracellular transport pathways, ultimately leading to the release of cytotoxic payloads within the cancer cell. This mechanism enhances the specificity of the therapy, ensuring that the cytotoxic drugs are delivered precisely to the target cells while minimizing exposure to healthy tissues. An antigen that facilitates efficient internalization is therefore essential for maximizing the therapeutic potential of ADCs in cancer treatment.
The target antigens of currently approved ADC drugs are typically proteins that are specifically overexpressed by cancer cells. These targets include HER2, Trop2, Nectin4, and EGFR in solid tumors, as well as CD19, CD22, CD33, CD30, BCMA, and CD79b in hematological malignancies. The selection of these antigens has been driven by advancements in oncology and immunology research. Over time, there has been a shift from targeting traditional tumor cell antigens to including targets within the tumor microenvironment, such as those found in the extracellular matrix and vasculature. This expansion in target selection has broadened the potential applications of ADCs in cancer therapy.
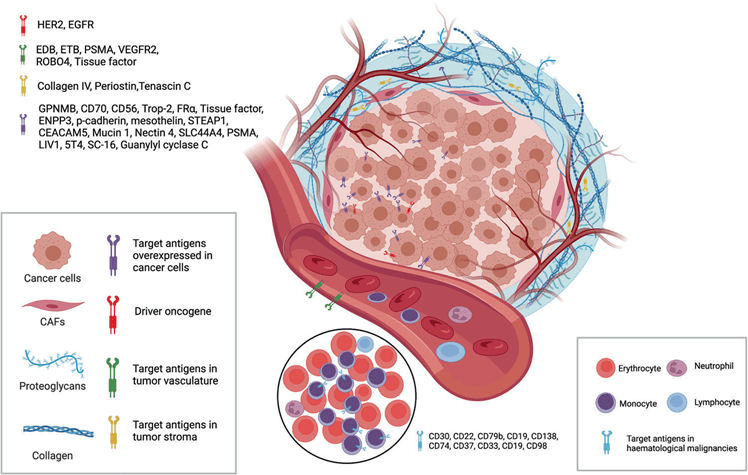
Figure 2. ADC drug target selection (tumor cells and tumor microenvironment)
ADC targets approved for marketing
Since the FDA approved the first ADC drug, Mylotarg® (gemtuzumab ozogamicin), in 2000, a total of 14 ADC drugs have been approved worldwide for treating hematological malignancies and solid tumors as of 2024. These 14 drugs target 12 specific antigens: HER2, CD22, BCMA, CD33, CD30, CD79b, Nectin-4, EGFR, CD19, Tissue Factor (TF), FRα, and Trop2.
| Number | Drug name | Company | Target | Cytotoxic drugs | Connector | DAR | Approval time |
|---|---|---|---|---|---|---|---|
| 1 | Mylotarg | Pfizer | CD33 | calicheamicin | Acid hydrolysis of hydrazone bonds and disulfide bonds | 2-3 | 2000.05.17 |
| 2 | Adcetris | Seattle | CD30 | MMAE | MC-VC-PABC | 4 | 2011.08.19 |
| 3 | Kadcyla | Roche | HER2 | DM1 | MCC | 3.5 | 2013.02.22 |
| 4 | Besponsa | Pfizer | CD22 | calicheamicin | Acid hydrolysis of hydrazone bonds and disulfide bonds | 5-6 | 2017.06.28 |
| 5 | Polivy | Roche | CD79b | MMAE | MC-VC-PABC | 3.5 | 2019.06.10 |
| 6 | Padcev | Seattle | Nectin-4 | MMAE | MC-VC-PABC | 3.8 | 2019.12.18 |
| 7 | Enhertu | Daiichi Sankyo | HER2 | Dxd | MC-GGFG | 7-8 | 2019.12.20 |
| 8 | Trodelvy | Immunomdecls | Trop-2 | SN38 | Acid cleaves carbonate bonds | 7.6 | 2020.04.22 |
| 9 | Blenrep | GSK | BCMA | MMAE | Uncut MC | 4 | 2020.08.05 |
| 10 | Akalux | Rakuten Medical | EGFR | IRDye700DX | N/A | 1.3-3.8 | 2020.09.25 |
| 11 | Zynlonta | ADC Therapeutics | CD19 | PBD | PEG8-VA-PABC | 2.3 | 2021.04.23 |
| 12 | Vidicil | Rongchang Biology | HER2 | MMAE | MC-VC-PABC | 4 | 2021.06.08 |
| 13 | Tlvdak | Genmab/Sesgen | TF | MMAE | MC-VC-PABC | 4 | 2021.09.20 |
| 14 | Elahere | ImmunoGen | ERα | DM4 | SPDB | 3 | 2022.11.14 |
ADC target research and development status
Statistics indicate that over 800 ADC drugs are currently in various stages of development globally. These drugs target a range of popular antigens, including HER2, Trop2, EGFR, c-Met, B7-H3, HER3, Nectin-4, CLDN18.2, and PDL1. These targets are considered mature and have shown promise in developing ADC therapies. The diversity of targets reflects ongoing efforts to expand the utility and effectiveness of ADCs in treating various cancers. This broad development pipeline underscores the growing interest and investment in ADC research and development worldwide.
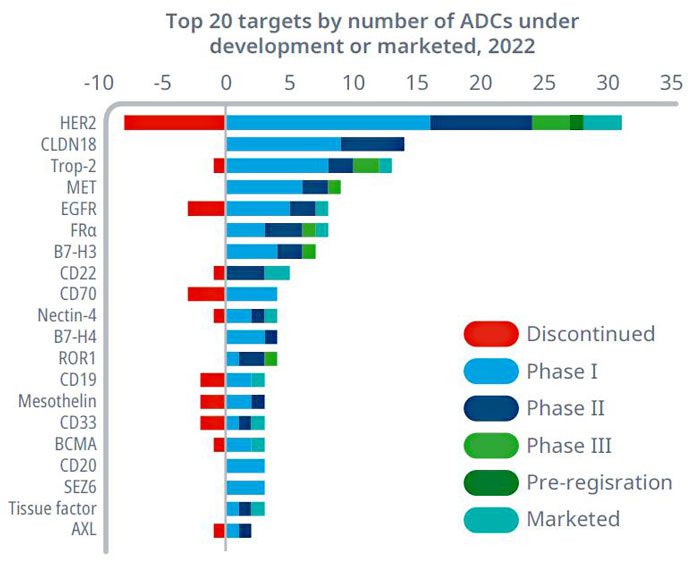
Figure 3. Global distribution of ADC targets under development (top 20) (as of 2022)
Common targets of ADC drugs in the field of lung cancer
The exploration of ADC drugs in the field of lung cancer mainly targets HER2, HER3, Trop2, MET, CEACAM5, B7-H3 and other targets (Figure 4), and has shown great application potential.
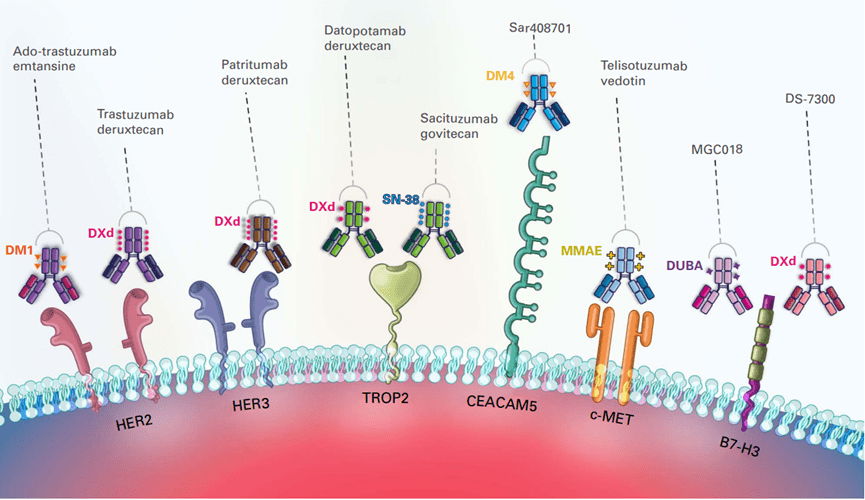
Figure 4. Overview of lung cancer ADC drug targets
HER2 target
HER2, a receptor tyrosine kinase (RTK), is a therapeutic target for non-small cell lung cancer (NSCLC). Unlike other HER family members with higher receptor turnover rates, HER2 is less prone to internalization and degradation. This unique characteristic allows HER2 to remain activated on the cell membrane for extended periods, making it an ideal target for ADC development. By targeting HER2, ADCs can deliver cytotoxic payloads directly to NSCLC cells with high specificity, potentially enhancing treatment efficacy while minimizing off-target effects. The distinctive properties of HER2 highlight its significance in ADC research and its potential to improve outcomes for NSCLC patients.
HER3 target
HER3, a member of the receptor tyrosine kinase (RTK) HER family, is often expressed in non-small cell lung cancer (NSCLC), with rates reaching up to 83%. Its role in NSCLC is significant, as HER3 heterodimerization can lead to resistance to EGFR tyrosine kinase inhibitors (TKIs). This resistance mechanism occurs by boosting the activation of HER3-dependent oncogenic signaling pathways. The high prevalence of HER3 expression in NSCLC underscores its importance as a potential therapeutic target. Developing strategies to target HER3, particularly in combination with other therapies, could potentially overcome resistance and improve outcomes for NSCLC patients.
Trop2 target
Trop2 is a transmembrane glycoprotein belonging to the epithelial cell adhesion molecule (EpCAM) family. It is widely expressed in most tumors but only sporadically found in normal tissues. This unique expression pattern makes Trop2 an attractive target for cancer therapy, as it allows for selective targeting of tumor cells while sparing healthy tissues, potentially minimizing off-target effects.
CEACAM5 target
Carcinoembryonic antigen-associated cell adhesion molecules (CEACAMs) are a family of cell surface glycoproteins. Specifically, CEACAM5 is found to be expressed in multiple tumor types, including lung cancer. This targeted expression makes CEACAM5 an appealing candidate for cancer therapy, offering the potential to precisely target tumor cells while minimizing damage to healthy tissues. This specificity could lead to reduced off-target effects and enhanced treatment effectiveness, improving overall outcomes for patients with these cancers.
MET targets
MET is a tyrosine kinase receptor that binds to hepatocyte growth factor. In non-small cell lung cancer (NSCLC), approximately 30%-50% of cases exhibit MET overexpression. Additionally, MET amplification occurs in 1.5% of cases, while MET exon 14 skipping mutations are present in 3% of cases. These alterations in MET are major drivers of cancer progression in NSCLC.
B7-H3 target
B7-H3, also known as CD276, is a significant immune checkpoint molecule belonging to the B7 family of proteins. While its specific ligand remains unclear, B7-H3 is notably highly expressed in various cancer cells. This overexpression in cancer suggests that B7-H3 may play a crucial role in immune regulation within the tumor microenvironment, making it a potential target for cancer immunotherapy.
In the field of lung cancer, along with the previously mentioned targets, ongoing clinical development is also focusing on other targets such as EGFR, AXL, and PVRL4. These targets represent promising avenues for the development of novel therapies aimed at improving outcomes for patients with lung cancer. Each target offers unique opportunities for targeted treatment approaches, highlighting the diverse and evolving landscape of lung cancer research and therapy development.
Conclusion
To summarize, the ADC field is experiencing intensifying competition, with a significant issue being the homogeneity of target selection. Most targets are currently in the stage of clinical efficacy verification. The future direction of exploration lies in optimizing target selection and differentiating target advantages across various dimensions, including technology and indications. This approach will be crucial for maximizing the potential of ADCs in cancer therapy and overcoming challenges related to target selection and efficacy verification.




