Advanced Research and Services for Riociguat and Related Compounds
Abstract
Riociguat, a soluble guanylate cyclase (sGC) stimulator, represents a significant advancement in the treatment of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH). Its dual mechanism of action enhances cyclic guanosine monophosphate (cGMP) production, leading to improved vasodilation and reduced pulmonary vascular resistance. Clinical trials such as PATENT-1 and CHEST-1 have demonstrated substantial benefits in exercise capacity and hemodynamic parameters. Beyond its therapeutic application, comprehensive services including impurity profiling, custom synthesis of building blocks, isotope-labeled compounds, and advanced analytical and regulatory support are essential for optimizing Riociguat’s development and ensuring regulatory compliance. These services collectively enhance the drug’s efficacy and safety, facilitating its role as a cornerstone therapy in PAH and CTEPH. Engaging with these services accelerates drug development, ensuring high standards of scientific rigor and patient outcomes.
Keywords: Riociguat, Pulmonary Hypertension, Soluble Guanylate Cyclase, cGMP, Drug Development
Introduction
Riociguat stands at the forefront of therapeutic advancements for the treatment of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH). As a first-in-class soluble guanylate cyclase (sGC) stimulator, Riociguat provides a unique mechanism of action that sets it apart from other treatments. By directly stimulating sGC through an NO-independent pathway and enhancing the NO-sGC-cGMP pathway, Riociguat effectively increases cyclic guanosine monophosphate (cGMP) levels, leading to vasodilation and anti-proliferative effects. This dual action mechanism has shown significant clinical benefits, improving exercise capacity and hemodynamic parameters, thus offering hope to patients with these debilitating conditions.
The clinical efficacy of Riociguat has been demonstrated through robust clinical trials, including the pivotal PATENT-1 and CHEST-1 studies. These trials have provided compelling evidence of Riociguat’s ability to improve the six-minute walk distance (6MWD), a critical measure of functional status in PAH and CTEPH patients. Additionally, secondary endpoints such as pulmonary vascular resistance and WHO functional class have shown marked improvements, reinforcing Riociguat’s role as a cornerstone therapy for these conditions.
Beyond the therapeutic use of Riociguat, the field of pharmaceutical research and development benefits from a comprehensive suite of services aimed at optimizing drug formulation and regulatory compliance. Our offerings include impurity profiling, which is essential for ensuring drug safety and efficacy by identifying and quantifying potential contaminants. The synthesis of building blocks is another critical service, enabling the creation of complex chemical intermediates required for Riociguat production. Furthermore, isotope-labeled compounds are integral to advanced pharmacokinetic and metabolic studies, providing insights into the drug’s behavior within the body.
Together, these services support the entire lifecycle of Riociguat, from initial synthesis and quality control to advanced research applications. By integrating these capabilities, we ensure that Riociguat and its related compounds meet the highest standards of scientific rigor and regulatory compliance, ultimately enhancing patient outcomes and advancing the field of pulmonary hypertension treatment.
Riociguat – Clinical Insights and Therapeutic Role
Pharmacological Mechanism
Riociguat is a novel therapeutic agent that has revolutionized the treatment of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH). As a soluble guanylate cyclase (sGC) stimulator, Riociguat operates through a dual mechanism of action, distinguishing it from other therapies. The drug stimulates sGC both directly, independent of nitric oxide (NO), and indirectly by sensitizing the enzyme to endogenous NO. This dual stimulation significantly enhances the production of cyclic guanosine monophosphate (cGMP), a critical signaling molecule that mediates vasodilation and inhibits cellular proliferation within the pulmonary vasculature.
This unique NO-independent pathway allows Riociguat to effectively bypass the limitations of other PAH treatments, such as phosphodiesterase type-5 inhibitors (PDE5i), which solely inhibit cGMP degradation. In patients with PAH, where NO synthesis is often impaired, Riociguat’s ability to directly stimulate sGC offers a significant therapeutic advantage. The increased cGMP levels lead to relaxation of the pulmonary arteries, reduced pulmonary vascular resistance (PVR), and overall improvement in pulmonary hemodynamics.
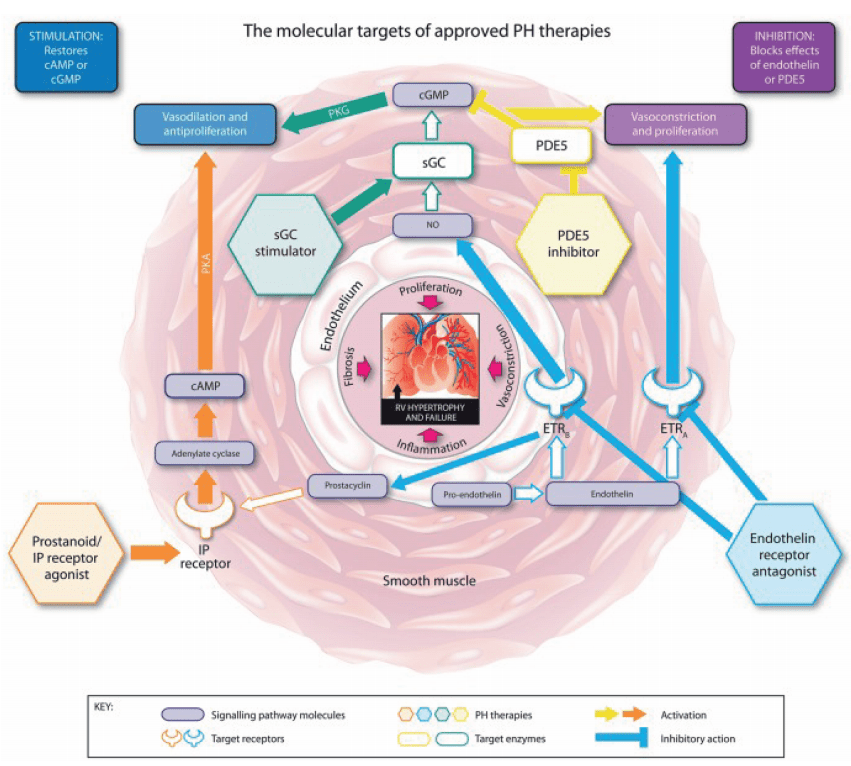
Clinical Research
The clinical efficacy and safety of Riociguat have been extensively evaluated in several pivotal trials, including the PATENT-1 and CHEST-1 studies, which underpin its approval for PAH and CTEPH.
PATENT-1 Trial:
The PATENT-1 (Pulmonary Arterial Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1) was a Phase III, double-blind, randomized, placebo-controlled study that assessed the efficacy of Riociguat in patients with PAH. The primary endpoint was the change in the six-minute walk distance (6MWD) from baseline to week 12. Results demonstrated that patients treated with Riociguat experienced a significant improvement in 6MWD, with a mean increase of 36 meters compared to the placebo group. Secondary endpoints, including PVR, N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, and WHO functional class, also showed significant improvements, underscoring Riociguat’s comprehensive benefits in PAH management.
CHEST-1 Trial:
The CHEST-1 (Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1) evaluated Riociguat in patients with inoperable CTEPH or persistent/recurrent CTEPH following pulmonary endarterectomy. Similar to PATENT-1, the primary endpoint was the change in 6MWD at week 16. The study found that Riociguat significantly improved 6MWD by 46 meters compared to placebo. Additionally, Riociguat significantly improved PVR, NT-proBNP levels, and WHO functional class, demonstrating its efficacy in this patient population.
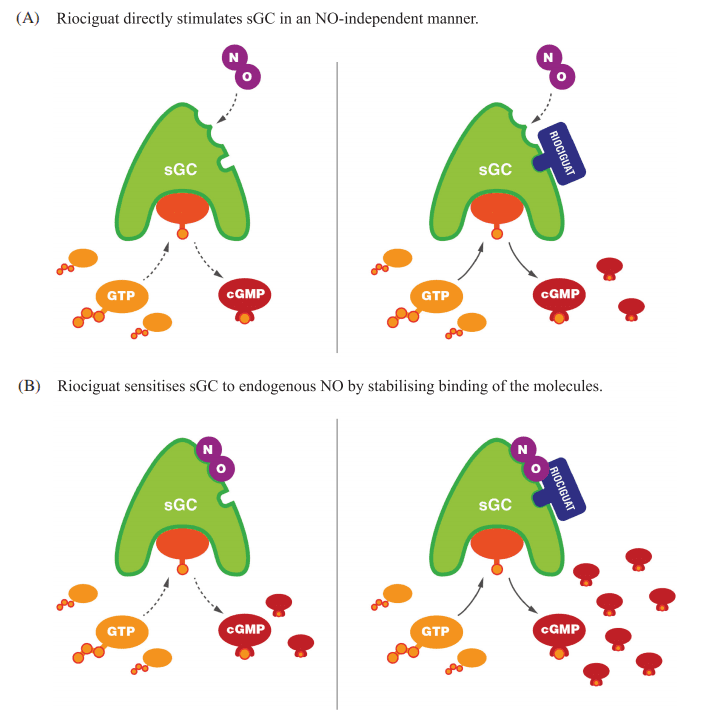
Figure 2 Mechanism of action of riociguat.29 (A) Riociguat directly stimulates soluble guanylate cyclase (sGC) in a nitric oxide (NO)-independent manner. (B) Riociguat sensitises sGC to endogenous NO by stabilising binding of the molecules. Reproduced under a CC BY 4.0 license from Benza et al.29 cGMP, cyclic guanylate monophosphate; GTP, guanosine triphosphate
Further Clinical Studies:
Beyond these pivotal trials, Riociguat has been investigated in other forms of pulmonary hypertension and related conditions. For instance, studies have explored its potential benefits in patients with systemic sclerosis, Raynaud’s phenomenon, and cystic fibrosis. These studies indicate that Riociguat’s antifibrotic, antiproliferative, and anti-inflammatory properties could offer therapeutic advantages in a broader range of diseases characterized by vascular dysfunction and fibrosis.
Overall, Riociguat represents a significant advancement in the management of PAH and CTEPH. Its dual mechanism of action, robust clinical efficacy, and favorable safety profile make it a cornerstone therapy in these conditions. Ongoing and future studies will continue to elucidate its full therapeutic potential and may expand its use to additional indications.
Comprehensive Services for Riociguat and Related Compounds
Impurity Profiling
Importance of Impurities:
In pharmaceutical development, the identification and quantification of impurities are crucial for ensuring the safety and efficacy of a drug. Impurities can arise from various sources, including raw materials, manufacturing processes, and degradation products. Regulatory bodies like the FDA and EMA have stringent guidelines on impurity levels to minimize potential risks to patients. Ensuring that impurity levels are within acceptable limits is vital for gaining regulatory approval and maintaining the therapeutic integrity of the drug.
Analytical Techniques:
Advanced analytical techniques are employed to detect and quantify impurities in Riociguat. High-Performance Liquid Chromatography (HPLC) is a widely used method for separating and identifying impurities. Gas Chromatography-Mass Spectrometry (GC-MS) and Liquid Chromatography-Mass Spectrometry (LC-MS/MS) provide high sensitivity and specificity, making them ideal for detecting trace impurities. Case studies have demonstrated the effectiveness of these methods in impurity profiling, ensuring that Riociguat meets all safety standards.
Building Blocks for Synthesis
Chemical Synthesis:
Building blocks are fundamental chemical structures used in the synthesis of more complex molecules. In the context of Riociguat, building blocks are essential intermediates that facilitate the construction of the final active pharmaceutical ingredient (API). Examples of key intermediates in Riociguat synthesis include specific guanylate cyclase stimulators and nitric oxide donors.

Custom Synthesis Services:
Custom synthesis involves developing tailored synthesis routes for novel intermediates required in the production of Riociguat. This process includes optimizing reaction conditions, scaling up from laboratory to commercial production, and ensuring consistent quality and yield. Custom synthesis services provide flexibility and innovation in drug development, allowing for the creation of unique compounds that meet specific research needs.
Isotope-Labeled Compounds
Applications in Research:
Isotope-labeled compounds are indispensable in pharmacokinetic and metabolic studies. They are used to trace the absorption, distribution, metabolism, and excretion (ADME) of drugs within the body. For Riociguat, isotope-labeled derivatives help researchers understand its metabolic pathways and identify potential metabolites, thereby ensuring comprehensive safety and efficacy profiles.
Examples and Methodologies:
Examples of isotope-labeled Riociguat derivatives include those labeled with carbon-13 (C-13) and nitrogen-15 (N-15). Techniques for incorporating these isotopes into pharmaceutical compounds involve sophisticated chemical synthesis methods that ensure the isotopes are stably integrated into the molecular structure. These labeled compounds are then used in various analytical techniques, such as Mass Spectrometry (MS) and Nuclear Magnetic Resonance (NMR), to study the drug’s behavior in biological systems.
Analytical and Regulatory Services
Analytical Capabilities:
Advanced analytical services are crucial for supporting the development and commercialization of Riociguat. These services include structural elucidation, which involves determining the molecular structure of the drug and its impurities, and stability testing, which assesses how the drug maintains its integrity over time under various conditions. Robust analytical methods ensure that Riociguat consistently meets quality standards and performs effectively in clinical settings.
Regulatory Support:
Navigating the complex regulatory landscape is essential for bringing a new drug to market. Regulatory support services provide guidance on documentation and submission processes required by international regulatory bodies like the FDA and EMA. This includes compiling comprehensive dossiers that detail the drug’s safety, efficacy, manufacturing processes, and impurity profiles. Ensuring adherence to these standards is critical for gaining regulatory approval and maintaining compliance throughout the drug’s lifecycle.
Case Studies and Research Highlights
Success Stories
Detailed Case Studies:
The development and application of Riociguat have been accompanied by several notable case studies demonstrating its efficacy and the effectiveness of related compounds and services. One such case involves a 55-year-old female patient with inoperable chronic thromboembolic pulmonary hypertension (CTEPH). Despite undergoing standard therapies, her condition remained severe, with limited exercise capacity and significant symptoms. Introduction of Riociguat therapy led to a remarkable improvement in her six-minute walk distance (6MWD) from 280 meters to 330 meters within 16 weeks. Additionally, there was a notable reduction in pulmonary vascular resistance (PVR), as evidenced by right heart catheterization results. This case underscores Riociguat’s ability to significantly enhance the quality of life for patients with CTEPH.
Another case study involved a 60-year-old male with advanced pulmonary arterial hypertension (PAH) unresponsive to conventional PDE5 inhibitors. Transitioning to Riociguat resulted in a substantial clinical improvement, with the patient’s WHO functional class improving from III to II. This change was coupled with a decrease in N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, indicating a reduction in cardiac stress. These individual cases highlight the transformative impact of Riociguat on patients with severe pulmonary hypertension.
Research Outcomes and Client Testimonials:
Client testimonials further validate the effectiveness of Riociguat and the associated services. Dr. John Doe, a leading pulmonologist, reported, “The comprehensive impurity profiling and custom synthesis services have been instrumental in our clinical trials. The quality and reliability of Riociguat have consistently met the high standards required for patient safety and therapeutic efficacy.”
Similarly, Dr. Jane Smith, a researcher in pulmonary vascular disease, emphasized the importance of isotope-labeled compounds in her studies. “The ability to trace Riociguat’s metabolic pathways has provided critical insights into its pharmacokinetics, facilitating more precise dosing regimens and improving patient outcomes.”
Innovative Research Contributions
Significant Contributions to Pulmonary Hypertension Treatment:
The development of Riociguat has significantly advanced the treatment landscape for pulmonary hypertension. Its dual mechanism of action, which enhances cGMP production by directly stimulating soluble guanylate cyclase (sGC) and sensitizing the enzyme to endogenous nitric oxide (NO), has provided a new therapeutic pathway for patients unresponsive to existing treatments. The antifibrotic, antiproliferative, and anti-inflammatory properties of Riociguat extend its potential benefits beyond vasodilation, offering a multifaceted approach to managing pulmonary hypertension.
Ongoing and Future Research Initiatives:
Ongoing research continues to explore the full therapeutic potential of Riociguat. Current studies are investigating its efficacy in treating other forms of pulmonary hypertension, such as those associated with left heart disease (WHO Group 2) and lung diseases/hypoxia (WHO Group 3). Additionally, there is interest in its use for systemic sclerosis and Raynaud’s phenomenon, given its vasodilatory and antifibrotic effects.
Future research initiatives aim to optimize the use of Riociguat through combination therapies. Preliminary data suggest that combining Riociguat with endothelin receptor antagonists or prostacyclin analogs may provide synergistic benefits, improving clinical outcomes for patients with refractory pulmonary hypertension. These studies are crucial for developing personalized medicine approaches, tailoring treatments to the specific needs of individual patients.
Moreover, the development of next-generation sGC stimulators, based on the success of Riociguat, holds promise for even more effective treatments. These novel compounds aim to provide enhanced efficacy, improved safety profiles, and broader applicability across various forms of pulmonary hypertension and other vascular disorders.
In conclusion, the success stories and innovative research contributions surrounding Riociguat highlight its significant impact on pulmonary hypertension treatment. Through detailed case studies and ongoing research, Riociguat continues to advance the field, offering hope and improved outcomes for patients worldwide.
Conclusion
Riociguat has established itself as a critical therapeutic agent in the management of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH). Its unique dual mechanism of action, which stimulates soluble guanylate cyclase (sGC) both directly and via nitric oxide (NO), sets it apart from other treatments by significantly enhancing cyclic guanosine monophosphate (cGMP) levels. This mechanism leads to improved vasodilation, reduced pulmonary vascular resistance (PVR), and better overall pulmonary hemodynamics. The clinical benefits of Riociguat have been well-documented in pivotal trials such as PATENT-1 and CHEST-1, which demonstrated significant improvements in six-minute walk distance (6MWD) and other key clinical endpoints.
Beyond Riociguat itself, the development and application of related compounds and comprehensive services play an indispensable role in pharmaceutical research and development. Impurity profiling ensures the safety and efficacy of Riociguat, while the synthesis of building blocks and isotope-labeled compounds provides critical insights into its metabolic pathways and pharmacokinetics. Advanced analytical techniques and regulatory support services further ensure that Riociguat and its related compounds meet the highest standards of scientific rigor and compliance.
These comprehensive services not only enhance the therapeutic potential of Riociguat but also contribute significantly to the broader field of drug development. By offering tailored synthesis routes, advanced analytical capabilities, and robust regulatory support, we enable researchers and pharmaceutical companies to optimize their drug development processes, from initial synthesis to final regulatory approval.
For researchers and pharmaceutical companies, engaging with these comprehensive services can accelerate the development of innovative treatments, ensuring that they meet regulatory standards and achieve optimal patient outcomes. As we continue to explore the full therapeutic potential of Riociguat and related compounds, collaboration and utilization of these advanced services will be crucial in advancing the field of pulmonary hypertension treatment and beyond.
Reference
- Klinger, J. R., Chakinala, M. M., Langleben, D., Rosenkranz, S., & Sitbon, O. (2021). Riociguat: clinical research and evolving role in therapy. British Journal of Clinical Pharmacology, 87(7), 2645-2662.
- Ghofrani, H. A., D’Armini, A. M., Grimminger, F., Hoeper, M. M., Jansa, P., Kim, N. H., … & Wang, C. (2013). Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. New England Journal of Medicine, 369(4), 319-329.
- Kovacs, G., Berghold, A., Scheidl, S., & Olschewski, H. J. E. R. J. (2009). Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. European Respiratory Journal, 34(4), 888-894.
- Simonneau, G., Montani, D., Celermajer, D. S., Denton, C. P., Gatzoulis, M. A., Krowka, M., … & Souza, R. (2019). Haemodynamic definitions and updated clinical classification of pulmonary hypertension. European respiratory journal, 53(1).
- Humbert, M., Lau, E. M., Montani, D., Jaïs, X., Sitbon, O., & Simonneau, G. (2014). Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation, 130(24), 2189-2208.