Advancements in Breast Cancer Treatment: Exploring FDA-Approved Drugs, Nanotechnology, and Future Directions
Abstract
Breast cancer remains a significant global health challenge, with over 2.26 million cases diagnosed in 2020 alone. This blog post explores the advancements in breast cancer treatment, focusing on FDA-approved drugs, nanotechnology, and future directions. FDA-approved treatments for breast cancer are classified into cytotoxic drugs, targeted therapies, and hormonal therapies, each with specific mechanisms of action and therapeutic applications. Nanotechnology has revolutionized drug delivery, offering promising solutions to enhance drug efficacy and reduce side effects through advanced formulations like nanoparticles, liposomes, and nanoemulsions. The future of breast cancer treatment is poised for significant advancements, driven by personalized medicine, immunotherapy, combination therapies, and the integration of artificial intelligence and big data analytics. These innovations hold great promise for improving patient outcomes and advancing therapeutic options, ultimately aiming to reduce the global burden of breast cancer and enhance the quality of life for patients.
Introduction to Breast Cancer and FDA-Approved Treatments
Breast cancer remains one of the most prevalent and formidable challenges in global healthcare. As the most diagnosed solid cancer, its impact is profound, with approximately 2.26 million cases reported in 2020 alone. The significance of early detection and the relentless pursuit of innovative treatments cannot be overstated, given that breast cancer accounts for a substantial percentage of cancer-related mortalities among women worldwide. The World Health Organization (WHO) has highlighted this concern, with initiatives like the Global Breast Cancer Initiative aiming to reduce global breast cancer mortality by 2.5% annually from 2020 to 2040. This ambitious goal underscores the importance of continuous research and development in breast cancer therapies.
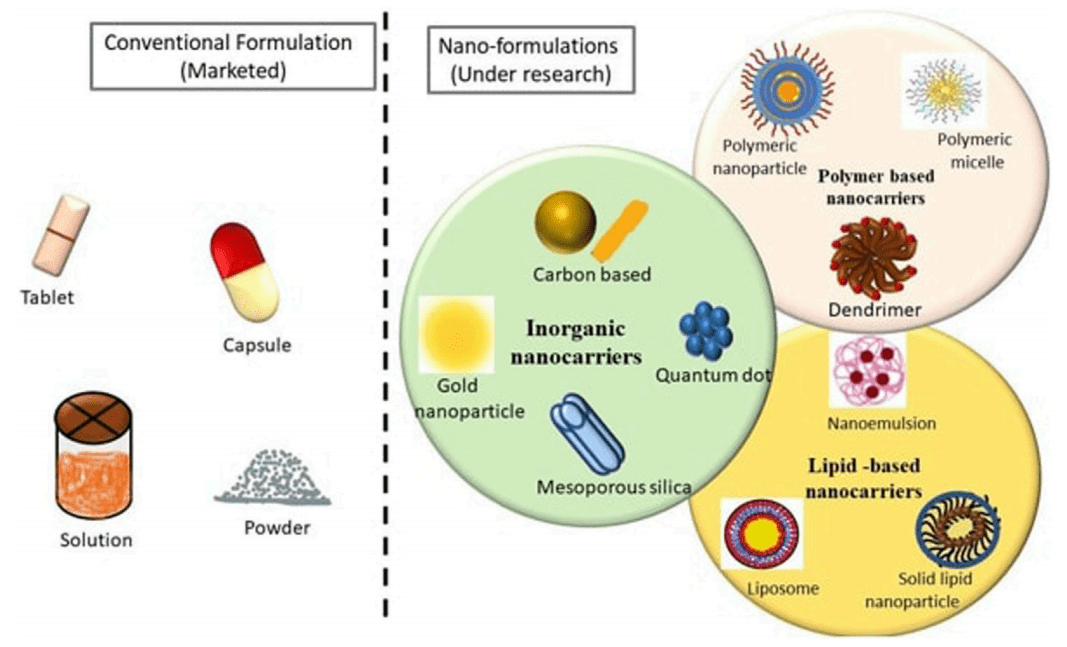
Figure 1 Pictorial representation of Conventional and Novel formulations of various FDA approved anticancer Drugs used for Breast Cancer Indications.
Breast cancer treatment has evolved significantly over the years, with a wide range of FDA-approved drugs available. These drugs are classified based on their mechanisms of action, including cytotoxic drugs, targeted therapies, and hormonal therapies. Cytotoxic drugs, such as Cyclophosphamide and Methotrexate, work by interfering with cell division and promoting apoptosis. Targeted therapies, like Trastuzumab and Lapatinib, focus on specific molecular targets associated with cancer cell growth. Hormonal therapies, including Tamoxifen and Fulvestrant, aim to block hormone receptors that fuel cancer progression.
Additionally, the development of novel drug formulations is a significant area of research. Nanotechnology-based formulations, for instance, offer promising alternatives by enhancing drug delivery and reducing side effects. Nanoparticles, liposomes, and nanoemulsions are examples of these advanced formulations that improve the bioavailability and targeting of therapeutic agents.
The ongoing advancements in breast cancer treatments highlight the critical role of continuous research and innovation. By understanding the landscape of FDA-approved drugs and their formulations, healthcare professionals can better tailor treatment strategies to improve patient outcomes. This comprehensive approach is essential for advancing therapeutic options and ultimately combating the global burden of breast cancer.
Classification and Mechanism of FDA-Approved Drugs
Breast cancer treatments encompass a diverse array of FDA-approved drugs, each classified based on its mechanism of action. Understanding these classifications is crucial for tailoring treatment strategies to individual patient needs and improving therapeutic outcomes. The primary categories include cytotoxic drugs, targeted therapies, and hormonal therapies, each playing a unique role in combating breast cancer.
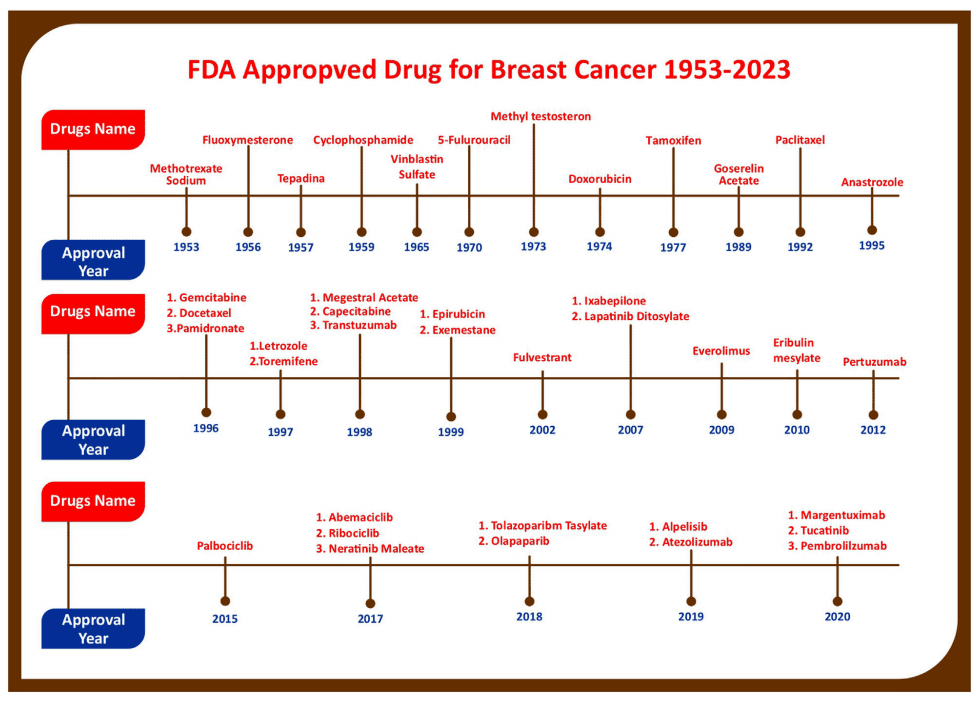
Figure 2 Figure showing progress in breast cancer treatment research as year wise account of anticancer drugs which are approved for various Breast Cancer Indications by US-FDA
Cytotoxic Drugs:
Cytotoxic drugs, also known as chemotherapy agents, work by targeting rapidly dividing cells, a hallmark of cancer. These drugs interfere with cell division and promote apoptosis (programmed cell death). Examples include Cyclophosphamide, which is an alkylating agent that inhibits DNA replication, and Methotrexate, an antimetabolite that inhibits dihydrofolate reductase, essential for DNA synthesis. Despite their effectiveness, cytotoxic drugs often come with significant side effects due to their impact on healthy, rapidly dividing cells.
Targeted Therapies:
Targeted therapies represent a more precise approach to cancer treatment. These drugs are designed to specifically target molecular abnormalities within cancer cells. Trastuzumab (Herceptin) is a monoclonal antibody that binds to the HER2 receptor, overexpressed in some breast cancers, thereby inhibiting cell proliferation. Lapatinib, a tyrosine kinase inhibitor, blocks the HER2 and EGFR pathways, hindering tumor growth. These therapies offer the advantage of fewer side effects compared to traditional chemotherapy, as they spare normal cells.
Hormonal Therapies:
Hormonal therapies are effective for hormone receptor-positive breast cancers, which rely on hormones like estrogen and progesterone for growth. Tamoxifen, a selective estrogen receptor modulator (SERM), blocks estrogen receptors on breast cancer cells, preventing their growth. Fulvestrant, a selective estrogen receptor degrader (SERD), not only blocks but also degrades the estrogen receptor, leading to more effective inhibition of cancer cell growth. Aromatase inhibitors, such as Letrozole, reduce estrogen levels by blocking the aromatase enzyme, crucial for estrogen synthesis in postmenopausal women.
Novel Drug Formulations:
The development of novel drug formulations is a significant focus in breast cancer research. Nanotechnology-based formulations, for example, offer promising improvements in drug delivery. Nanoparticles, liposomes, and nanoemulsions enhance the bioavailability and targeting of drugs, minimizing side effects and maximizing therapeutic efficacy. These advanced formulations hold the potential to revolutionize breast cancer treatment by providing more effective and personalized therapeutic options.
The classification and mechanisms of FDA-approved drugs for breast cancer highlight the advancements in therapeutic strategies. By leveraging these diverse approaches, healthcare professionals can offer more effective and tailored treatments, improving patient outcomes and quality of life.
Nanotechnology in Breast Cancer Treatment
Nanotechnology has revolutionized the field of breast cancer treatment, offering innovative solutions to the limitations of conventional drug delivery systems. By employing nanoscale materials, researchers have developed advanced formulations that enhance the effectiveness and specificity of therapeutic agents while minimizing adverse side effects. This section explores the role of nanotechnology in breast cancer treatment, focusing on various nanotechnology-based drug delivery systems.
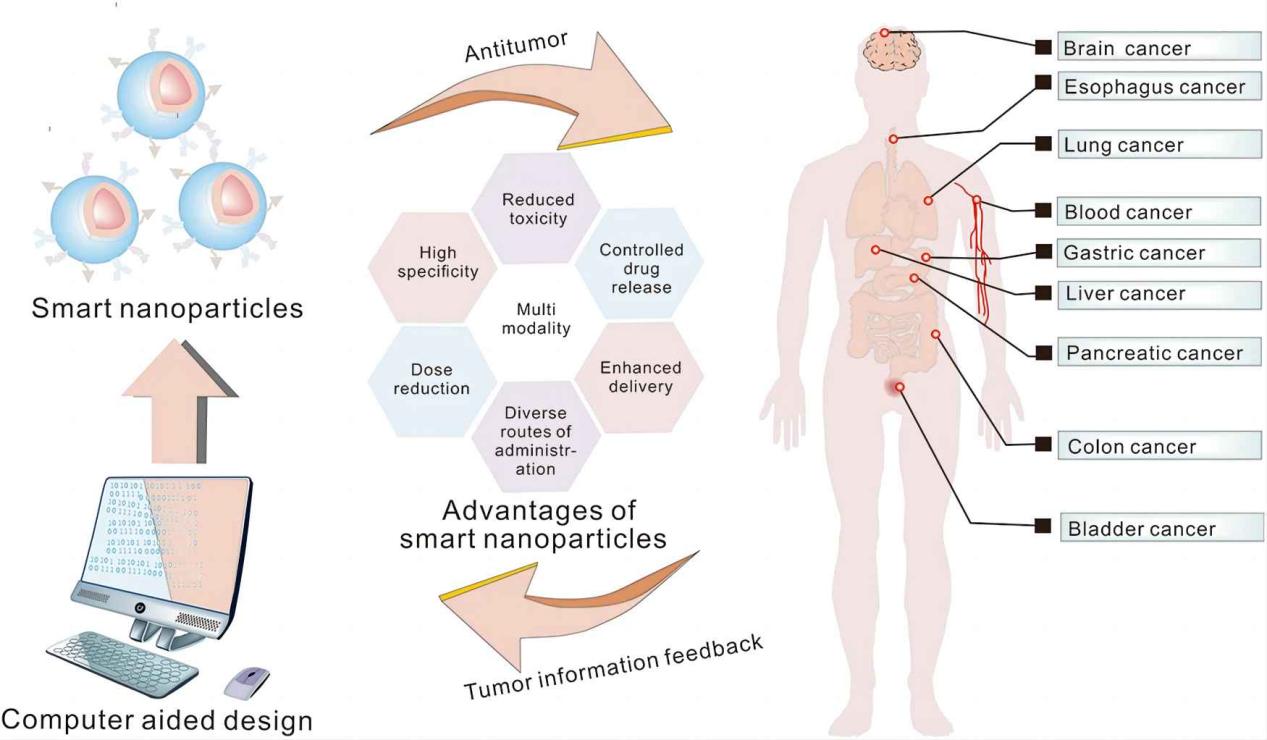
Figure 3 Schematic illustration of smart nanoparticles for cancer treatment
Introduction to Nanotechnology-Based Drug Delivery:
Nanotechnology involves manipulating materials at the nanoscale to create particles with unique properties that enhance drug delivery. In breast cancer treatment, nanotechnology-based formulations offer several advantages over traditional methods. These include improved solubility of hydrophobic drugs, targeted delivery to tumor sites, controlled drug release, and reduced systemic toxicity. Nanoparticles, liposomes, and nanoemulsions are some of the most promising nanocarriers in this field.
Nanoparticles:
Nanoparticles are solid colloidal particles ranging from 10 to 1000 nanometers in size, used to encapsulate drugs. They can be engineered to improve drug stability, bioavailability, and targeting. For instance, polymeric nanoparticles can be designed to release drugs in response to specific stimuli such as pH or temperature, providing controlled and sustained drug delivery. Metallic nanoparticles, like gold or silver, offer unique optical and thermal properties that can be utilized for imaging and hyperthermia treatment in addition to drug delivery.
Liposomes:
Liposomes are spherical vesicles composed of lipid bilayers, capable of encapsulating both hydrophilic and hydrophobic drugs. They enhance drug solubility and stability, and their biocompatibility reduces the risk of toxicity. Liposomal formulations can be modified with surface ligands to target specific cancer cells, increasing the accumulation of the drug at the tumor site while sparing healthy tissues. Liposomal Doxorubicin, for example, is a well-known formulation used to treat breast cancer, providing prolonged circulation time and reduced cardiotoxicity.
Nanoemulsions:
Nanoemulsions are fine oil-in-water or water-in-oil emulsions with droplet sizes ranging from 20 to 200 nanometers. They offer high drug-loading capacity and enhanced bioavailability for both hydrophilic and hydrophobic drugs. Nanoemulsions can improve the penetration of drugs into cancer cells and provide controlled release, making them highly effective in breast cancer treatment. Their stability and ease of preparation further add to their potential as a drug delivery system.
Future Prospects:
The future of breast cancer treatment lies in the continued development and optimization of nanotechnology-based drug delivery systems. Research is focused on creating multifunctional nanocarriers that combine therapeutic, diagnostic, and imaging capabilities, paving the way for personalized and precision medicine. The integration of nanotechnology with molecular and genetic profiling of tumors will enable the development of targeted therapies that are tailored to the unique characteristics of each patient’s cancer.
Nanotechnology offers a promising avenue for enhancing breast cancer treatment, providing innovative solutions to improve drug delivery and patient outcomes. The ongoing research and development in this field hold the potential to revolutionize cancer therapy, making treatments more effective and less toxic.
Detailed Review of Key FDA-Approved Drugs
Breast cancer treatment involves a variety of FDA-approved drugs, each with specific mechanisms of action and clinical applications. This section provides a detailed review of some key FDA-approved drugs, highlighting their therapeutic efficacy, formulations, and novel drug delivery systems.
Cyclophosphamide:
Cyclophosphamide is a nitrogen mustard alkylating agent approved in 1959. It works by interfering with DNA replication, thus inhibiting cancer cell growth. Cyclophosphamide is used in combination with other chemotherapy agents, such as Methotrexate and Fluorouracil, for greater efficacy. It is available in both oral and injectable forms, with the latter being more common in clinical practice. The primary side effects include bone marrow suppression, alopecia, and hemorrhagic cystitis. Recent research focuses on developing pH-sensitive nanoparticles to enhance its anticancer capacity and reduce off-target effects.
Methotrexate Sodium:
Methotrexate Sodium, an antimetabolite, inhibits dihydrofolate reductase, essential for DNA synthesis. Approved for breast cancer in 1959, it is effective against various cancers, including head and neck cancers and leukemias. Methotrexate is available as a subcutaneous injection, with doses ranging from 7.5 mg to 25 mg. Nanotechnology has advanced its delivery through lipid nanoemulsions, nanogels, and solid lipid nanoparticles, improving its therapeutic profile by increasing its mean residence time in blood circulation.
Trastuzumab (Herceptin):
Trastuzumab, approved in 1998, is a monoclonal antibody targeting the HER2 receptor overexpressed in some breast cancers. By binding to HER2, it inhibits cell proliferation and induces an immune response leading to cancer cell death. Trastuzumab is administered intravenously and is available in weekly and three-weekly regimens. Despite its effectiveness, concerns about cardiotoxicity and resistance persist. Research is ongoing to incorporate Trastuzumab in novel formulations like liposomal drugs to enhance its efficacy and reduce adverse effects.
Tamoxifen:
Tamoxifen, a selective estrogen receptor modulator (SERM), has been a cornerstone in breast cancer treatment since its approval. It blocks estrogen receptors on breast cancer cells, preventing their growth. Tamoxifen is used for hormone receptor-positive breast cancers, particularly in premenopausal women. It is available in oral tablet form. While effective, Tamoxifen’s long-term use is associated with increased risks of endometrial cancer and thromboembolic events. Efforts are being made to develop nanoformulations to improve its safety profile and therapeutic efficacy.
Doxorubicin Hydrochloride:
Doxorubicin, an anthracycline antibiotic, intercalates DNA base pairs and inhibits topoisomerase II, leading to DNA strand breakage. Approved for metastatic breast cancer, it is administered as an intravenous injection. Doxorubicin’s significant side effect is cardiotoxicity, which limits its long-term use. Liposomal formulations like Doxil have been developed to mitigate these adverse effects, providing targeted delivery and reducing toxicity.
The detailed review of these key FDA-approved drugs highlights the advancements in breast cancer treatment. Continuous research and development in drug formulations and delivery systems are crucial for improving therapeutic outcomes and reducing side effects. The integration of nanotechnology and personalized medicine will further enhance the effectiveness of breast cancer therapies, offering hope for better patient care.
Future Directions and Conclusion
The future of breast cancer treatment is poised for significant advancements, driven by continuous research and innovation in drug development and delivery systems. As the understanding of breast cancer biology deepens, new therapeutic targets and strategies are emerging, promising more effective and personalized treatments.
Ongoing Research and Future Prospects:
One of the most exciting areas of research is the development of personalized medicine. By leveraging genetic and molecular profiling of tumors, treatments can be tailored to the specific characteristics of each patient’s cancer. This approach not only improves efficacy but also reduces the risk of adverse side effects. Additionally, advancements in nanotechnology continue to play a crucial role. Researchers are exploring multifunctional nanocarriers that can deliver drugs, provide imaging capabilities, and target cancer cells with high precision. These innovations aim to enhance drug delivery, reduce toxicity, and overcome resistance mechanisms.
Immunotherapy:
Immunotherapy is another promising frontier in breast cancer treatment. Drugs that enhance the body’s immune response to cancer, such as immune checkpoint inhibitors, have shown efficacy in other cancer types and are now being investigated for breast cancer. Combining immunotherapy with existing treatments like chemotherapy and targeted therapy could lead to more comprehensive and effective treatment regimens.
Combination Therapies:
Combination therapies are gaining traction as a means to tackle cancer from multiple angles. By using drugs that target different pathways and mechanisms, the likelihood of overcoming resistance increases. This approach is particularly beneficial in treating advanced and metastatic breast cancer, where single-agent therapies often fall short. Clinical trials are continuously testing various combinations to identify the most effective protocols.
Artificial Intelligence and Big Data:
The integration of artificial intelligence (AI) and big data analytics in oncology is transforming how breast cancer is diagnosed and treated. AI can analyze vast amounts of data to identify patterns and predict responses to treatment, aiding in the development of personalized treatment plans. Moreover, AI-driven diagnostic tools can improve the accuracy of early detection, leading to better outcomes.
In conclusion, the landscape of breast cancer treatment is rapidly evolving, with numerous innovative approaches on the horizon. The advancements in personalized medicine, nanotechnology, immunotherapy, and AI hold great promise for improving patient outcomes. Continuous research and collaboration among scientists, healthcare providers, and patients are essential to translate these innovations into clinical practice. As we move forward, the goal remains to provide more effective, less toxic, and personalized treatments for breast cancer, ultimately reducing its global burden and improving the quality of life for patients.
Reference
- Chaurasia, M., Singh, R., Sur, S., & Flora, S. J. S. (2023). A review of FDA approved drugs and their formulations for the treatment of breast cancer. Frontiers in Pharmacology, 14, 1184472.
- Jain, K. K., & Jain, K. K. (2008). The handbook of nanomedicine (Vol. 404). Totowa:: Humana Press.
- Agrawal, Y. O., Mahajan, U. B., Mahajan, H. S., & Ojha, S. (2020). Methotrexate-loaded nanostructured lipid carrier gel alleviates imiquimod-induced psoriasis by moderating inflammation: formulation, optimization, characterization, in-vitro and in-vivo studies. International Journal of Nanomedicine, 4763-4778.
- Sun, L., Liu, H., Ye, Y., Lei, Y., Islam, R., Tan, S., … & Cai, L. (2023). Smart nanoparticles for cancer therapy. Signal transduction and targeted therapy, 8(1), 418.