Advancements in CD73 Inhibitors: Pioneering Cancer Immunotherapy
Abstract
CD73, known scientifically as ecto-5′-nucleotidase, is a crucial enzyme involved in the immunosuppressive microenvironment of tumors. By converting AMP to adenosine, CD73 regulates immune suppression, facilitating tumor evasion from immune surveillance. The development of small molecular inhibitors targeting CD73 offers a promising avenue for cancer immunotherapy, aiming to restore immune system efficacy against tumors. This paper reviews recent progress in the development of CD73 inhibitors, discussing their molecular design, clinical trials, challenges, and future perspectives.
Introduction
The immune system is critical in managing and eradicating cancer cells by recognizing and destroying them. However, this defense mechanism can be undermined by enzymes like CD73, which facilitate a tumor-protective immunosuppressive microenvironment. CD73 does this by converting AMP to adenosine, a molecule that significantly weakens immune surveillance and response mechanisms against tumor cells. Given its central role in dampening immune attacks on tumors, CD73 has become a prime target in the development of specific inhibitors. These inhibitors aim to block adenosine production, thereby restoring the immune system’s ability to fight cancer effectively. Recent advances in this area have led to the identification and development of various CD73 inhibitors, highlighting the enzyme’s potential as a therapeutic target in oncology. This research promises to enhance the efficacy of immunotherapies and possibly improve outcomes for patients with various cancers.
CD73: Role and Mechanism
CD73, also recognized as ecto-5′-nucleotidase, is a glycosylphosphatidylinositol (GPI)-anchored cell surface protein that plays a pivotal role in the tumor microenvironment by hydrolyzing extracellular nucleotides into adenosine. This conversion is critical as adenosine is a potent immunosuppressive agent, effectively reducing the ability of the immune system to identify and attack tumor cells. The widespread expression of CD73 on various cell types, including tumor cells and different immune cells such as T cells and B cells, underscores its importance. By promoting adenosine production, CD73 aids in tumor growth and helps tumors evade immune surveillance.
Moreover, CD73 contributes to the progression of cancer by supporting tumor growth, facilitating metastasis, and enhancing the tumor’s resistance to conventional cancer treatments like chemotherapy and radiotherapy. These roles are mediated through adenosine’s effects on various signaling pathways that promote cancer cell proliferation, inhibit apoptosis (programmed cell death), and enhance the capacity of cancer cells to spread to other parts of the body. Thus, CD73 is not only a marker of poor prognosis in several cancer types but also a potential target for therapeutic intervention aimed at countering the immunosuppressive environment and improving treatment efficacy.
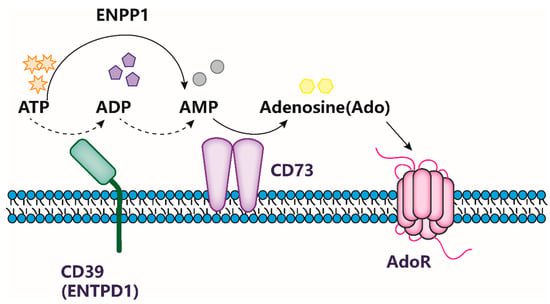
Fig.1 CD73 accelerates the accumulation of ADO
Development of CD73 Inhibitors
The development of CD73 inhibitors marks a significant advancement in cancer immunology, initiated by the recognition of CD73’s crucial role in promoting an immunosuppressive microenvironment conducive to tumor growth. Researchers designed small molecular inhibitors that specifically target the active site of CD73, effectively blocking its ability to convert AMP into adenosine. This interruption is vital because adenosine plays a key role in suppressing immune responses against tumors. By inhibiting CD73, these molecules aim to diminish adenosine-mediated immunosuppression, thereby potentially enhancing the effectiveness of immune checkpoint inhibitors and other therapeutic strategies in oncology. These inhibitors are being explored not only as standalone treatments but also in combination with other therapies to improve patient outcomes by reinstating the immune system’s ability to combat cancer more effectively. This strategy represents a promising approach to overcoming the challenges of immune evasion by tumors.
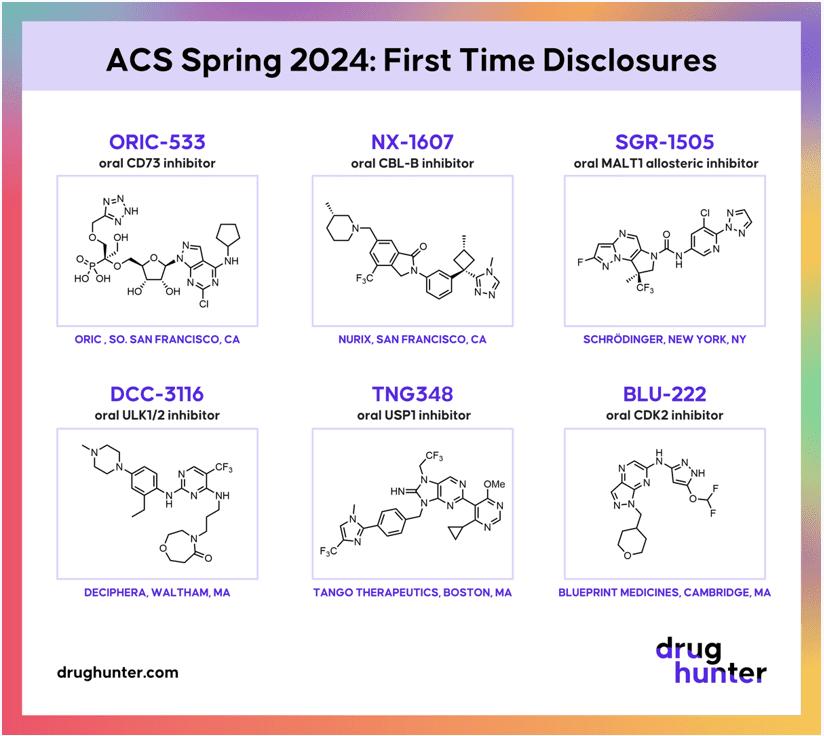
Fig.2 Recent discoveries of CD73 inhibitors
Several CD73 inhibitors are currently being evaluated in clinical trials. Here are some of the notable ones:
ORIC-533 – This small-molecule CD73 inhibitor is being tested in Phase 1b clinical trials specifically for people with multiple myeloma.
Oleclumab (MEDI9447) – An anti-CD73 monoclonal antibody, Oleclumab is being investigated in combination therapies, such as with Durvalumab and standard radiation therapy for locally advanced non-small cell lung cancer.
NZV930 – This is another anti-CD73 antibody undergoing clinical trials in combination with other immunotherapeutic agents.
PT199 – A monoclonal antibody against CD73, PT199 is designed to inhibit both soluble and membrane-bound CD73, enhancing the responsiveness of anti-tumor immune cells to checkpoint immunotherapies.
These inhibitors target the adenosinergic checkpoint by either blocking the enzymatic activity of CD73 or preventing its expression, thereby potentially reversing the immunosuppressive conditions fostered by tumors.
Current Progress and Clinical Trials
Several small molecule CD73 inhibitors, initially synthesized and evaluated through preclinical studies, have shown promising potential in reducing tumor growth and enhancing immune responses. These positive outcomes have led to the progression of some inhibitors into clinical trials, where their safety, tolerability, and efficacy are being rigorously tested in human subjects. These clinical trials are crucial as they explore the utility of CD73 inhibitors both as standalone treatments and in combination with existing immunotherapies. The goal is to determine the most effective therapeutic strategies that leverage these inhibitors to combat various cancers, ultimately establishing optimized treatment protocols that could significantly improve patient outcomes.
Challenges in CD73 Inhibitor Development
Although the development of CD73 inhibitors has made significant strides, numerous challenges impede their path to clinical success. A major concern is ensuring that these inhibitors are both highly specific and potent without causing undesirable off-target effects, which can complicate treatment outcomes. Moreover, the chemical attributes of these inhibitors, such as stability, solubility, and bioavailability, present additional hurdles. These properties are crucial for effective drug delivery and action within the body, influencing the overall therapeutic efficacy. Addressing these challenges is essential for advancing these inhibitors through clinical trials and into medical use, necessitating further research and development to refine their formulation and enhance their performance in inpatient treatments.
Future Perspectives
The future of CD73 inhibitor development is optimistic, yet hinges on focused advancements across multiple fronts. Enhancing molecular design to increase specificity and potency is paramount to minimize adverse effects and maximize efficacy. Additionally, the exploration of innovative delivery mechanisms could revolutionize how these inhibitors are administered, potentially improving patient compliance and treatment effectiveness. Conducting thorough clinical trials is essential to validate safety and efficacy across diverse patient populations. Furthermore, the identification of biomarkers that can predict individual responses to CD73 inhibitors is crucial. Such biomarkers would enable personalized treatment plans, optimizing therapeutic outcomes and ensuring that patients receive the most effective and tailored therapies available.
Conclusion
CD73 inhibitors are emerging as a revolutionary force in cancer immunotherapy, offering a promising avenue for transforming the treatment landscape for various cancers. These inhibitors target the immunosuppressive mechanisms tumors use to evade immune detection, thereby reactivating the immune system’s ability to fight cancer. As research into CD73 inhibitors advances, they are poised to become essential to comprehensive cancer treatment protocols, especially when used with other immunotherapies. The synergistic potential of combining CD73 inhibitors with checkpoint inhibitors or other immune modulators could lead to more robust and sustained anti-tumor responses. Current studies and future explorations continue to build a dynamic pipeline of developments that promise to shift traditionally immunosuppressive tumor microenvironments to immunopermissive ones. This shift is crucial for enabling the immune system to mount effective responses against cancer cells, potentially leading to better patient outcomes and longer survival rates.
References
- Ge GH, Wang QY, Zhang ZH, Zhang X, Guo S, Zhang TJ, Meng FH. Small molecular CD73 inhibitors: Recent progress and future perspectives. Eur J Med Chem. 2024 Jan 15;264:116028. DOI: 10.1016/j.ejmech.2023.116028.
- Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Haskó G. Anti-CD73 in cancer immunotherapy: Awakening new opportunities. Trends Cancer. 2016 Feb;2(2):95-109. DOI: 10.1016/j.trecan.2016.01.003.
- Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002 Oct;110(7):993-1002. DOI: 10.1172/JCI0215918.
- Hay CM, Sult E, Huang Q, Mulgrew K, Fuhrmann SR, McGlinchey KA, Hammond SA, Rothstein R, Rios-Doria J, Poon E, Holoweckyj N, Durham NM, Leow CC, Diedrich G, Damschroder M, Herbst R, Hollingsworth RE, Sachsenmeier KF. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology. 2016;5(7):e1176294. DOI: 10.1080/2162402X.2016.1176294.
- Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev. 2017 Jan;276(1):121-144. DOI: 10.1111/imr.12528.
- Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017 Dec;17(12):709-724. DOI: 10.1038/nrc.2017.86.
- Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK. Adenosine receptor 2A blockade increases the efficacy of anti-PD-1 through enhanced antitumor T-cell responses. Cancer Immunol Res. 2015 May;3(5):506-517. DOI: 10.1158/2326-6066.CIR-14-0211.
- Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer. 2018;6(1):57. DOI: 10.1186/s40425-018-0360-8.




