Advances in Genitourinary Tissue Engineering: Insights from Cutting-Edge Research
Abstract
Genitourinary tissue engineering represents a rapidly evolving field that aims to develop innovative solutions for the treatment of complex urethral and vaginal disorders. Traditional treatment methods often involve invasive surgeries with significant risks of complications and limited long-term success. Recent advances, particularly the self-assembly technique, have shown great promise in overcoming these challenges by creating patient-specific tissue constructs that closely mimic the natural extracellular matrix and tissue architecture. This technique, which allows cells to produce their own extracellular matrix, offers a biocompatible and functionally superior alternative to conventional grafts. The integration of emerging technologies such as bioprinting and the use of induced pluripotent stem cells (iPSCs) further enhances the potential of genitourinary tissue engineering, paving the way for personalized regenerative medicine. As research continues to advance, these innovative approaches hold the promise of significantly improving patient outcomes and quality of life.
Understanding Genitourinary Tissue Engineering
Despite the significant advances in medicine of the last decades, a lot of illnesses are still challenging. Indeed, the treatments themselves can be insufficient, or their side effects may negatively impact the patient’s quality of life. From congenital anomalies to various injuries or illnesses that may develop, the list goes on and can range from a simple annoyance to a deadly threat. Affectations of the genitourinary tissues are no exception in this respect and can significantly contribute to undermining the social and emotional life of individuals.
Genitourinary tissue engineering represents a transformative frontier in regenerative medicine, focusing on the development of biological substitutes to restore or replace the function of damaged or diseased tissues in the genitourinary system. This system includes vital organs such as the urethra, bladder, and vagina, which can be affected by a range of congenital anomalies, injuries, and diseases.
Tissue engineering been considerably developed to offer new and innovative solutions to old problems. Tissue engineering consists of the in vitro reconstruction of tissues or organs for the replacement/repair and 3D models for fundamental research. These conventional approaches can lead to complications, such as tissue rejection, limited availability of donor material, and significant post-operative morbidity. Genitourinary tissue engineering seeks to overcome these challenges by developing engineered tissues that are biocompatible, reduce the risk of rejection, and provide a more sustainable solution for long-term patient care.
The basis of tissue engineering protocols is to create scaffolds, which can have a synthetic or natural origin, seeded or not with cells. Advanced techniques, such as the self-assembly method, are being explored to enhance the efficacy of these engineered tissues by enabling the creation of more complex and functional tissue structures without the need for exogenous materials.
Challenges in Treating Urethral and Vaginal Disorders
Urethral and vaginal disorders are prevalent and often lead to significant discomfort and health issues, making effective treatment critical. The urethra, a vital part of the urinary system, can be affected by conditions such as urethral stricture and hypospadias. Urethral strictures are typically caused by injury, infection, or inflammation and result in the narrowing of the urethra, which obstructs urine flow. Hypospadias, a congenital condition where the urethral opening is not located at the tip of the penis, affects one in every 250 male births. Both conditions often require surgical intervention to restore normal function, but the success rates can vary, and complications such as scarring or infection are common.
Similarly, the vagina can be affected by congenital and acquired disorders that require complex management. Vaginal agenesis, also known as Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome, is a congenital condition where the vagina is underdeveloped or absent, affecting approximately 1 in 4,500 female births. Other conditions, such as vaginal stenosis or strictures, can occur due to cancer treatments, particularly radiation therapy, or as a result of chronic inflammatory conditions. Traditional treatments for these conditions include surgical reconstruction using skin grafts or intestinal segments. However, these methods are often associated with significant complications, including scarring, infection, and limited success in restoring normal function.
The limitations of these conventional treatments highlight the need for innovative approaches in tissue engineering. New techniques, such as the development of bioengineered tissues, offer the potential to improve patient outcomes by providing more effective and less invasive treatment options. These advancements could revolutionize the management of urethral and vaginal disorders, reducing the need for repeated surgeries and improving the quality of life for patients.
The Self-Assembly Technique: A Game-Changer
The self-assembly technique represents a significant advancement in the field of tissue engineering, particularly for genitourinary applications. This innovative method stands out because it does not rely on exogenous biomaterials, which can often lead to complications such as immune rejection and foreign body reactions. Instead, the self-assembly technique utilizes the natural ability of cells to produce their own extracellular matrix (ECM), creating tissue constructs that closely mimic the native structure and function of human tissues.
In this process, cells are cultured in the presence of ascorbic acid, which promotes the secretion of collagen and other ECM components. Over time, these cells self-organize into cohesive tissue sheets that can be layered to form more complex structures. This approach is particularly advantageous in reconstructing tissues like the urethra and vagina, where the native ECM plays a critical role in maintaining tissue integrity and function.
One of the most compelling aspects of the self-assembly technique is its ability to produce tissues with high mechanical strength and excellent biocompatibility. These engineered tissues can be customized to match the specific requirements of the patient, potentially reducing the risk of complications and improving the overall success of reconstructive surgeries. Additionally, the self-assembly method allows for the creation of more intricate tissue architectures, which are essential for the proper function of complex organs.
As research in this area progresses, the self-assembly technique is poised to revolutionize tissue engineering, offering new hope for patients with severe genitourinary disorders. By providing a more natural and effective approach to tissue reconstruction, this method could significantly enhance the outcomes of surgical treatments and improve the quality of life for those affected by these conditions.
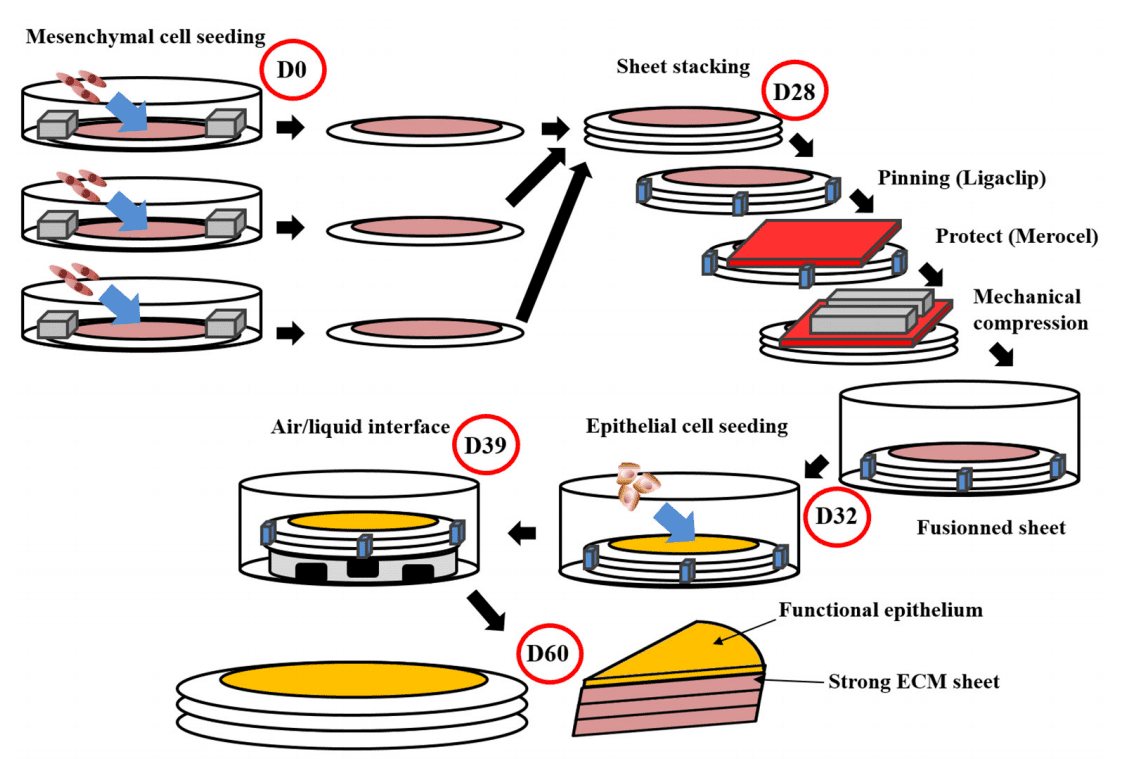
Revolutionizing Urethral and Vaginal Reconstruction
The self-assembly technique has opened new avenues in the reconstruction of complex tissues like the urethra and vagina, which have long posed significant challenges in regenerative medicine. Traditional methods for reconstructing these tissues often involve the use of grafts from other parts of the body, such as the oral mucosa or skin. However, these approaches are frequently associated with complications, including donor site morbidity, scarring, and limited tissue availability.
By contrast, the self-assembly technique offers a more sophisticated and patient-specific solution. In urethral reconstruction, for example, this method allows for the creation of tissue constructs that are structurally and functionally similar to the native urethra. These constructs can be tailored to fit the unique anatomical and physiological needs of the patient, potentially reducing the risk of complications such as strictures or fistulas. Early clinical trials have shown promising results, with patients experiencing improved urinary function and fewer postoperative issues.
Similarly, in vaginal reconstruction, the self-assembly technique has demonstrated significant potential. This method enables the development of vaginal tissue that closely replicates the natural environment, including the presence of essential extracellular matrix components and proper tissue elasticity. These engineered tissues have been successfully used in preclinical models, paving the way for future clinical applications. The ability to produce a functional neovagina using the patient’s own cells could greatly improve outcomes for individuals with congenital anomalies, cancer-related defects, or other conditions requiring reconstructive surgery.
As these techniques continue to evolve, the self-assembly approach is expected to play a pivotal role in advancing the field of genitourinary reconstruction. It not only promises better patient outcomes but also represents a major step forward in the development of more effective and reliable tissue engineering solutions.
The Future of Genitourinary Tissue Engineering
The field of tissue engineering can take potential alternatives to the constantly in adding need for tissue repair/replacement of urological organs. To do so, a ture challenge is to combine good mechanical properties while maintaining stem cell potential. It must also need adequate epithelium differentiation, and its degradation product should not imply adverse effects. It must be vascularised easily and not represent an immune risk for patients, while being functional as soon as the graft is in place. We can, therefore, better understand the significant obstacle that must be overcome.
Another exciting frontier is the use of stem cells and induced pluripotent stem cells (iPSCs) in genitourinary tissue engineering. These cells offer the potential to generate any type of tissue needed for repair or reconstruction. By harnessing the power of iPSCs, researchers may one day be able to create complete, autologous tissues and organs for transplantation, eliminating the risks associated with donor tissue rejection.
In conclusion, the future of genitourinary tissue engineering is bright, with ongoing research poised to bring about groundbreaking treatments that could transform the lives of patients suffering from debilitating genitourinary conditions. As these technologies mature, they hold the promise of not only improving patient outcomes but also making personalized and regenerative medicine a reality.
References
Langer, R., & Vacanti, J. P. (1993). Tissue engineering. Science, 260(5110), 920-926.




