Advances in Modern Immunology
Abstract
In recent decades, concurrent with the evolution of modern immunology, researchers have achieved significant breakthroughs in unraveling the cellular and molecular intricacies of immune regulation across various domains, including infectious diseases, allergic conditions, and neurological disorders. A deeper comprehension of the roles, interplay, and equilibrium among different immune cells and molecules promises to underpin effective scientific foundations and approaches for immune intervention, paving the way for novel treatments in the realm of immune-related illnesses.
Infectious Diseases
In the last century, five major global epidemics of infectious diseases have revolved around respiratory-associated viruses, notably influenza A and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In response to respiratory virus infections, the immune system triggers innate and adaptive responses, initiating antiviral immunity and a cascade of inflammatory reactions. An effective immune response aims to neutralize the virus, curb its invasion, eliminate infected target cells, maintain a delicate balance between inflammation and tissue repair, and ultimately ensure bodily homeostasis. Conversely, severe infections and excessive immune reactions can result in pathological damage, such as cytokine storms and organ inflammation disorders, leading to multi-organ tissue damage and functional impairment.
Following infection, the body’s pattern recognition receptors (PRRs), including Toll-like receptors (TLR), nucleotide-binding and oligomerization domain-like receptors (NLR), retinoic acid-inducible gene I-like receptors (RLR), and cyclic GMP-AMP synthase (cGAS), recognize pathogen-associated molecular patterns (PAMPs). This recognition activates downstream signaling pathways, induces inflammatory responses, stimulates innate immune cells, and initiates anti-infection immune responses. Additionally, soluble PRRs like ficolin, mannose-binding lectin (MBL), and lipopolysaccharide-binding protein (LBP) play crucial roles. For instance, ficolin gene deficiency can result in persistent lung infections, emphasizing its importance in lung anti-infection immunity. Ficolin A activates the extracellular complement pathway, protecting against pneumonia, while ficolin B promotes local lung inflammation recovery.
In severe influenza virus infections, ficolin A’s increased expression exacerbates pulmonary injury and overactivates the complement system, generating allergic toxins C3a and C5a.[1] Similar complement system overactivation has been observed in SARS-CoV-2-infected patients, accompanied by the presence of ficolin+SPP1+ inflammatory mononuclear macrophages and the secretion of pro-inflammatory cytokines like S100A8/A9, interleukin-1β, and interleukin-6. Notably, a novel nucleus-based PRR, hnRNPA2B1, has been identified, translocating to the cytoplasm to initiate antiviral innate immune responses.[2]
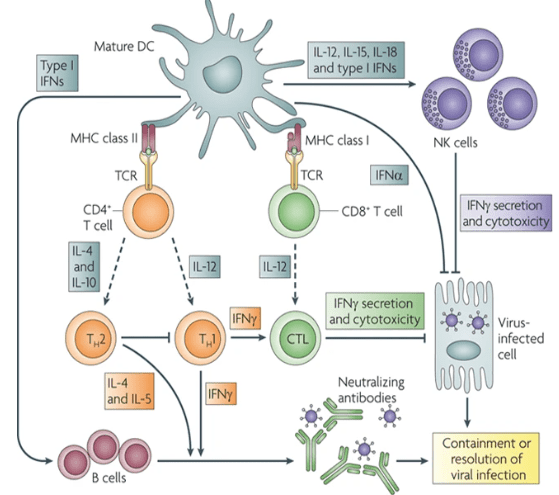
Figure 1. Infection and Immunology
Furthermore, a recently discovered activation mechanism dependent on physico-mechanical pressure, the PIEZO1-Ca2+ ion flow/AP-1-EDN1-HIF -1α-IL-1β/CXCL2 pathway, expands the role of non-classical PRRs in countering bacterial infections in the lungs. This intricate network of immune responses holds promise for better understanding and managing infectious diseases.
Cytokine storms, characterized by the release of various cytokines such as IL-6, IL-1β, IL-8, IL-17, G-CSF, GM-CSF, IP-10, and others, are implicated in the multi-tissue organ damage seen in severe influenza virus and SARS-CoV-2 infections. To combat COVID-19, the IL-6R neutralizing antibody tocilizumab has been employed to reduce mortality in hospitalized patients. Severe H1N1 infections have been shown to induce interferon-γ (IFN-γ) expression, and IFN-γ-neutralizing antibodies significantly enhance survival rates in infected mice. Additionally, IL-36γ plays a proinflammatory role, with elevated levels of IL-36γ and IL-36R observed in severe infection cases. These elevations promote IFN expression and STAT1 activation, contributing to respiratory epithelial cell apoptosis and autophagy inhibition early in the infection process.
Furthermore, studies have demonstrated that an increased proportion of lung γδT cells, particularly the Vγ4+γδT subgroup, is associated with IL-17A levels. Depletion of γδT or Vγ4+γδT cells reduces IL-17A levels, delays severe infection-induced pathological injury, and improves survival rates. Natural killer cells (NK) and cytotoxic T lymphocytes (CTL) contribute to cell pyroptosis through granzyme A-mediated cleavage of gasdermin B (GSDMB).
The proper functioning of pattern recognition receptors (PRR) and cytokines relies on the activation of their downstream signaling pathways, which are pivotal targets for immunotherapy. In a mouse model of H1N1 infection, activation of STAT1 and STAT3 was found to vary with time and dose. Moderate STAT1/3 activation was beneficial for antiviral immune responses in low-dose infections, whereas high-dose infections led to earlier and more intense STAT1/3 activation, cytokine storms, increased neutrophil infiltration, imbalanced macrophage polarization, and dysregulated inflammatory responses. Presently, the Janus kinase (JAK) inhibitor Baricitinib has shown promise as an immunomodulatory therapy for hospitalized COVID-19 patients [3].
Anaphylactic Diseases
Allergic diseases represent chronic conditions stemming from the body’s abnormal immune response to allergens, affecting multiple systems and organs. This encompasses disorders like allergic asthma, allergic rhinitis, allergic conjunctivitis, urticaria, and atopic dermatitis[4]. Over the past few decades, global industrialization, changes in environmental factors, dietary adjustments, and increased allergen exposure have led to a significant rise in the prevalence of allergic diseases. For instance, the incidence of asthma in developed countries can reach 8%, with approximately 4.2% of Chinese adults affected, while allergic rhinitis affects about 40% of the world’s population[5]. Consequently, the World Health Organization (WHO) has designated allergic diseases as a top priority for 21st-century research and prevention. Furthermore, comorbidity patterns among allergic diseases have made these conditions even more intricate.
With increasing research and attention on allergic diseases, there is a deeper understanding of their etiology, pathogenesis, and natural development processes. These diseases can manifest at any age, influenced by genetic factors, the body’s immune regulation, and the external environment. Environmental factors can elevate the risk for genetically susceptible individuals through gene-environment interactions[6]. Factors such as early hygiene practices, Westernized lifestyles replacing traditional ones, and a fast-paced way of life have been linked to the prevalence of allergies[7]. Different allergic disease phenotypes and endophenotypes may exhibit unique molecular mechanisms, associated biomarkers, and responses to biotherapy. Since the inception of the “hygiene hypothesis” by Strachan in 1989, numerous molecules related to allergic disease pathogenesis have been discovered, opening up new avenues for endotyping diseases associated with the microbiome. In recent years, type 2 and non-type 2 immune responses have emerged as the most common endophenotypes in allergic diseases, with subtypes like inflammasome subtypes, barrier subtypes, type 17, and mixed types also recognized.
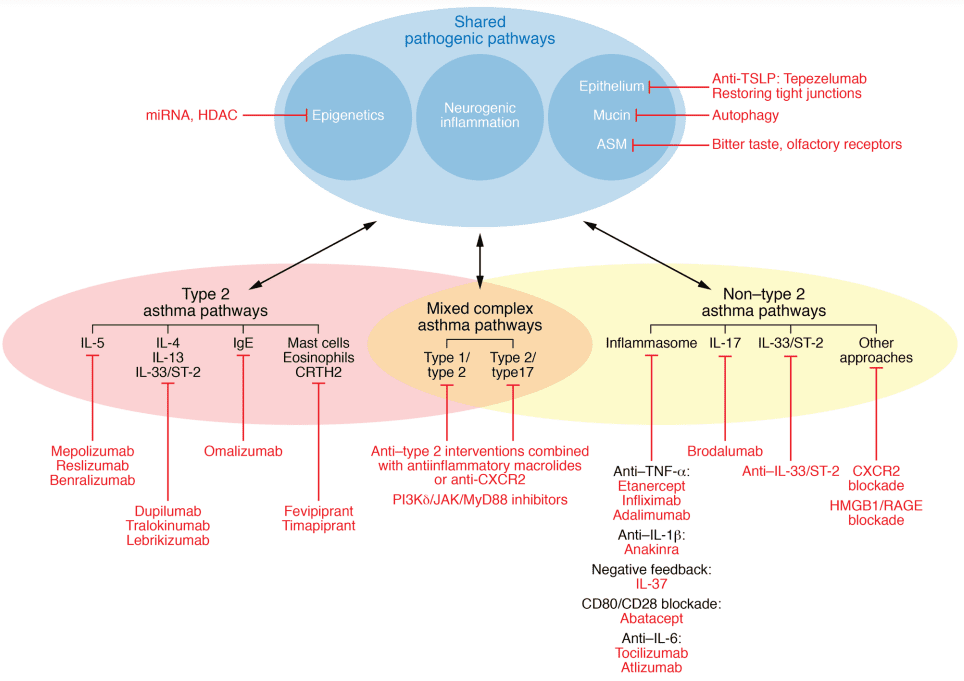
Figure 2. The dynamic and complex interaction between risk factors, disease phenotypes and endotypes, and expression modulators in allergic diseases in the context of precision medicine.[8]
In type 2 immune responses, allergens and pathogens entering the skin or mucosal epithelium are processed by antigen-presenting cells (APCs), leading to the differentiation of type 2 helper T cells (Th2). Epithelial-derived cytokines like IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) activate group 2 innate lymphoid cells (ILC2). Activated Th2 cells and ILC2s produce type 2 cytokines, including IL-4, IL-13, IL-5, and IL-9. Among these, IL-4 and IL-13 drive immunoglobulin E (IgE) class switching in B cells, resulting in allergen-specific IgE production. Allergen-specific IgE binds to the IgE Fc receptor I (FcεRI) on the surface of macrophages and basophils. IL-4, IL-9, and IL-13 promote mucus secretion through goblet cells, while IL-5 is involved in eosinophil recruitment. Consequently, many new biologic drugs target key elements in type 2 responses, such as IgE, IL-5, IL-4/IL-13, and TSLP. Meanwhile, subtypes of non-type 2 immune responses driven by TNF-α/NF-κB pathway overactivation often show limited responses to hormonal therapy, posing ongoing challenges for research into their mechanisms and the development of targeted drugs.
Nervous System Diseases
Neuroimmunity has emerged as a prominent area of research within immunology, focusing primarily on neuroimmune autoimmune diseases, acute brain injuries, and neurodegenerative conditions.
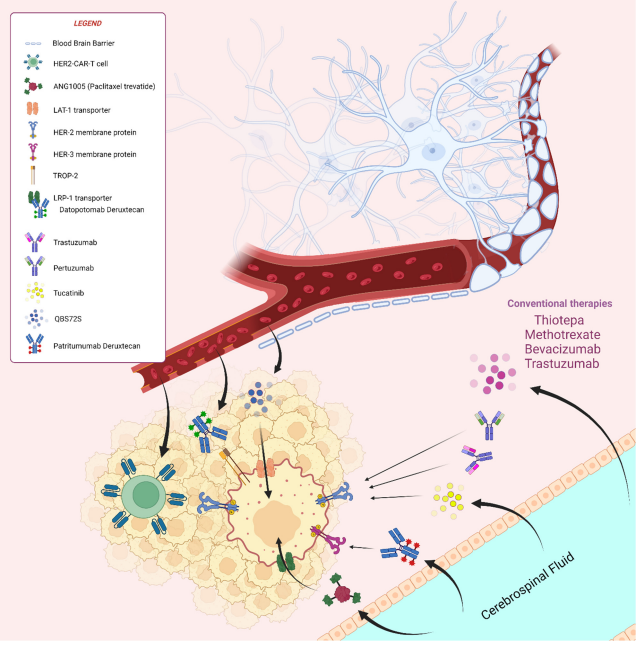
One notable autoimmune disease of the nervous system is multiple sclerosis (MS), characterized by chronic demyelination in the central nervous system. The current understanding attributes the induction of MS to a complex interplay of environmental and genetic factors, ultimately leading to an autoimmune response mediated by T lymphocytes targeting neuronal myelin antigens. Recent research has unveiled a critical aspect of MS pathogenesis, revealing that abnormal myeloid cell proliferation in the bone marrow plays a pivotal role in driving the disease’s progression. This process involves autoreactive T cells homing to the bone marrow via the chemokine CXCL12 and promoting myeloid cell generation through the CCL5-CCR5 signaling pathway. This, in turn, contributes to the autoimmune response by T cells and leads to inflammatory damage within the nervous system.Autoimmune encephalitis (AIE) is a category of acute or subacute inflammatory autoimmune diseases that result from interactions between the immune system and parenchymal tissue. It can be broadly classified into two types: infectious and non-infectious. The pathogenesis of AIE is intricately linked to the presence of anti-neuronal antibodies. These antibodies target various antigens, falling into three primary categories: antibodies against cell surface antigens (CSAab), antibodies against synaptic antigens (SYAab), and antibodies against intraneuronal antigens (INAab). Within these categories, numerous antigenic molecules have been identified as therapeutic targets for AIE. Examples include antibodies against the N-methyl-D-aspartate receptor (NMDAR), leucine-rich glioma inactivated protein 1 (LGI1), and the gamma-aminobutyric acid type B receptor (GABABR). Additionally, CSAab can activate the complement pathway, potentially leading to the lytic death of nerve cells.
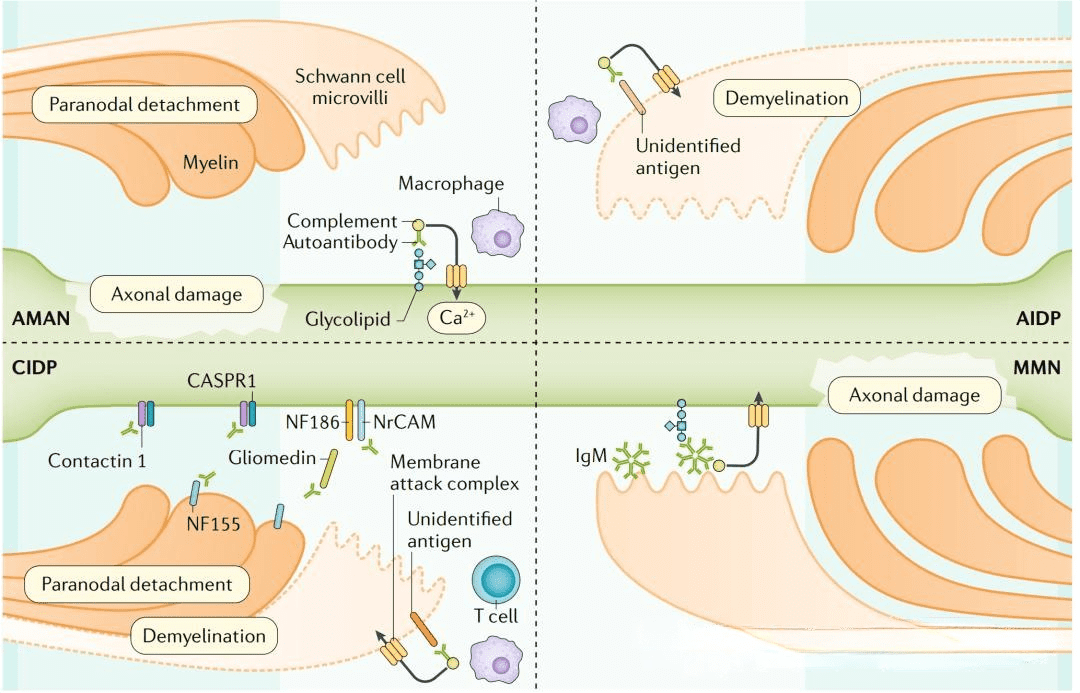
Figure 3. Autoimmune diseases of the nervous system
Acute brain injury, particularly in the form of stroke, is a severe cerebrovascular condition characterized by two main types: ischemic stroke and hemorrhagic stroke. Following a stroke, damaged nerve cells trigger both the innate and adaptive immune responses within the brain through the release of injury-related molecular pattern factors and other danger signals. This, in turn, exacerbates central nervous inflammation and injury. Microglia, pivotal mediators in this process, play a crucial role in shaping the pathological course of stroke.
Research has demonstrated that the transcription activator peroxisome proliferator-activated receptor-gamma (PPAR-γ) coactivator-1alpha (PGC-1α) can notably inhibit the production of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α in microglial cells in animal models of ischemic brain injury. This inhibition serves to mitigate the inflammatory microenvironment in the brain and prevent the progression of ischemic brain injury.
Clinical studies have also highlighted the importance of monitoring neuroinflammation in stroke patients. It has been observed that up to 32.0% of patients with mild stroke and transient ischemic attacks exhibited significantly elevated levels of hypersensitive C-reactive protein (hsCRP). Those with hsCRP levels exceeding 3 mg/L had a heightened risk of recurrent stroke within one year and experienced poorer functional outcomes. These findings underscore the direct impact of neuroinflammation on the pathological development and prognosis of stroke patients.
Neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and depression, represent significant challenges in the field of neurology and immunology.
Alzheimer’s disease (AD), characterized by severe memory decline and weakened learning abilities, has traditionally been associated with the deposition of amyloid β-protein (Aβ) and Tau protein phosphorylation, leading to extensive neuron loss[9]. Recent research suggests that AD is fundamentally an autoimmune disease occurring within the brain, triggered when the nervous system is damaged or infected by bacteria. This phenomenon is attributed to the high similarity between lipid molecules in bacterial cell membranes and neuronal cell membranes. Aβ, unable to distinguish between the two, mistakenly attacks neurons, resulting in chronic progressive neuronal loss and dysfunction. Furthermore, metabolic dysfunction in microglia involving glycolysis and histone lactation M2 isozyme type M2 (PKM2) can disrupt neurohomeostasis and promote inflammation, contributing to the development of Alzheimer’s disease.
Parkinson’s disease (PD), the second most common neurodegenerative disease after AD, is characterized by the death of dopaminergic neurons in the striatum and substantia nigra of the midbrain[10]. Excessive activation of the NOD-like receptor protein 3 (NLRP3) inflammasome in microglia can drive the pathological progression of PD. However, the inhibition of NLRP3 and cysteinyl aspartate-specific proteinase-1 (Caspase-1) can reverse the death of dopaminergic neurons and mitigate the onset of Parkinson’s disease.
Depression is closely associated with central nervous system immune disorders. Individuals with persistent depression often exhibit enhanced abnormalities in peripheral blood monocytes, lymphocytes, and neutrophils. Pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α are significantly upregulated in peripheral blood, brain tissue, and cerebrospinal fluid of depressed patients. These cytokines can influence neuron-glial cell interactions and impact synaptic plasticity. The protein programmed cell death 4 (PDCD4) negatively regulates brain-derived neurotrophic factor (BDNF) expression and is involved in chronic stress-induced anxiety and depression-like behavior. Minocycline, a small anti-depressive molecule, exerts its effects primarily by inhibiting the overactivation of T cells, neutrophils, and microglia, reducing the release of pro-inflammatory cytokines and increasing the release of anti-inflammatory cytokines. These insights into the immunological aspects of neurodegenerative diseases offer promising avenues for potential interventions and therapies.
Summary
The recent advancements in immunology have significantly contributed to our comprehensive understanding of various diseases. It has offered profound insights into the mechanisms underlying the onset and progression of diseases from an immunological perspective, and has also propelled the application of immunological theories and technologies in the prevention and treatment of a wide array of conditions. Moreover, the rapid development of multi-omics, big data, and systems biology has introduced powerful research tools that enable us to explore the intricate complexity and dynamic variations of immunological mechanisms across different diseases. The seamless integration of basic and clinical research is undeniably of great significance in shaping the future of immune disease diagnosis and treatment strategies. This collaborative approach, combining immunological insights with cutting-edge technologies and data-driven methodologies, promises to revolutionize the way we approach disease understanding, diagnosis, and treatment, ultimately leading to improved healthcare outcomes.