Advances in Orphan Drugs: From Rare Diseases to Promising Therapies
Abstract
Orphan drugs are medicines developed specifically to treat rare diseases (commonly known as “orphan diseases”). These diseases are considered rare because they affect a relatively small number of people. For the FDA, “supporting the development and evaluation of new therapies for rare diseases is of paramount importance.” According to data from the EMA, there are 5,000 to 8,000 different rare diseases. So far, there is no unique and clear definition of orphan diseases. In the United States, it is defined as a disease that affects fewer than 620 cases per million people; in Europe, it’s 500 cases per million people; in Japan, it’s 400 cases per million people; and in China, it’s 100 cases per million people. Due to their rarity, these diseases often receive limited attention from the pharmaceutical industry, making it financially unattractive for companies to invest in research and development for treatment methods.
The European Union and the United States have implemented legislation to incentivize the development of drugs for patients with rare diseases. Companies and other drug developers can apply for orphan drug designation. For the United States, if a drug meets specific criteria, the FDA will grant orphan drug designation. The benefits of orphan drug designation include eligibility for federal research funding, a 7-year period of market exclusivity, and up to 50% tax credit for qualifying clinical trials (until 2018). Starting from 2018, the U.S. government reduced the tax credit from 50% to 25%.
The evaluation of new therapies for rare diseases is a priority for the FDA/EMA. They can grant drugs or biologics orphan drug designations to prevent, diagnose, or treat rare diseases. Orphan drug designation ensures certain advantages in terms of tax incentives and a period of market exclusivity after approval. Most rare diseases are of genetic origin and can be life-threatening. Besides their genetic origins, orphan diseases can also be caused by parasitic, protozoal, bacterial infections, or even environmental toxins.
Orphan drug sales have significantly outpaced conventional pharmaceutical categories, with an annual growth rate of approximately 10% from 2005 to 2011. It is estimated that by 2026, the top-selling small molecule orphan drugs will include ibrutinib, elexacaftor/tezacaftor/ivacaftor, olaparib, ruxolitinib, venetoclax, acalabrutinib, and tafamidis. The projected sales for these orphan drugs in the United States and other regions of the world by 2026 are shown in Figure 1. The value of the top 10 “blockbuster” orphan drugs is estimated to be between 3 billion to 13 billion US dollars.
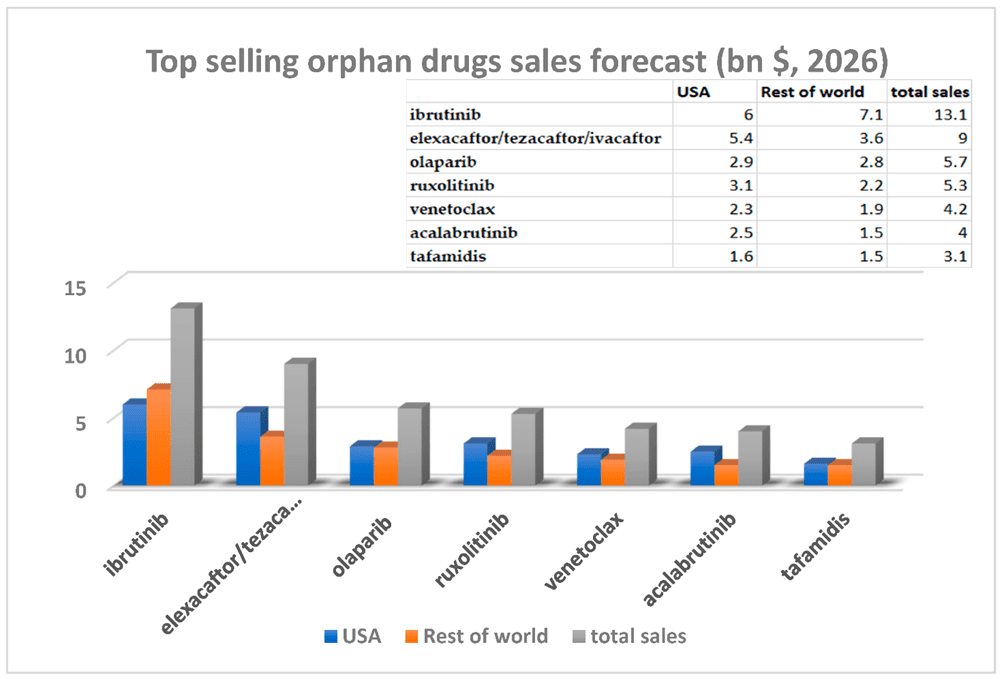
Fig 1. Estimated sales of blockbuster orphan drugs in 2026.
Ibrutinib:
It is a small molecule irreversible potent inhibitor of Bruton’s tyrosine kinase (BTK). It is a covalent-targeted drug, with its acrylamide structure acting as a covalent warhead, engaging in a Michael addition reaction with Cys481 on the target. It has demonstrated high activity against B-cell malignancies. Ibrutinib received FDA approval in November 2013 for the treatment of mantle cell lymphoma. In February 2014, it was also approved for the treatment of chronic lymphocytic leukemia, and later found use in patients with Waldenström macroglobulinemia.
In August 2017, Ibrutinib gained approval for the treatment of chronic graft-versus-host disease (cGVHD), a complication that can arise after stem cell transplantation. Notably, it was subsequently approved for use in children, making it the first FDA-approved therapy for cGVHD in children aged 1 year and older after failure of one or more systemic therapies.
Ibrutinib’s approval for multiple indications highlights its versatility and efficacy in addressing different B-cell malignancies and cGVHD, offering new treatment possibilities for patients with these challenging conditions. As further research continues, Ibrutinib’s role in oncology is likely to expand, potentially bringing hope to even more patients in need of effective therapies.

Fig 2. The structural formula of Ibrutinib.
Elexacaftor/Tezacaftor/Ivacaftor:
It is a groundbreaking medication that has transformed the treatment landscape for cystic fibrosis (CF). It is a combination drug therapy developed by Vertex Pharmaceuticals, and it received its first global approval in 2019 by the U.S. Food and Drug Administration (FDA).
Cystic fibrosis is a genetic disorder that affects the respiratory and digestive systems, causing the production of thick, sticky mucus that clogs the airways and leads to lung infections and digestive issues. Elexacaftor/Tezacaftor/Ivacaftor is designed to address the underlying genetic mutations that cause CF, specifically targeting those with the most common mutation, known as the F508del mutation.
The medication is a triple combination therapy consisting of three active components: elexacaftor, tezacaftor, and ivacaftor. Elexacaftor is a next-generation corrector, tezacaftor is a corrector, and ivacaftor is a potentiator. Together, they work synergistically to improve the function of the cystic fibrosis transmembrane conductance regulator (CFTR) protein on the cell surface, allowing for more effective chloride ion transport and thinner mucus production.
Clinical trials have shown that Elexacaftor/Tezacaftor/Ivacaftor significantly improves lung function and reduces the frequency of pulmonary exacerbations in patients with CF. It has been a game-changer for many individuals with cystic fibrosis, offering them a life-changing treatment option and significantly improving their quality of life.
While Elexacaftor/Tezacaftor/Ivacaftor represents a significant advancement in CF therapy, ongoing research continues to explore the potential of these and other combination therapies to address a broader range of CF mutations and improve outcomes for patients with this challenging condition.

Fig 3. The structural formula of Elexacaftor-Tezacaftor-Ivacaftor.
Olaparib:
It is a selective and potent inhibitor of poly (ADP-ribose) polymerase (PARP). PARP inhibitors represent a novel class of cancer therapies. Olaparib works by inhibiting the repair of damaged DNA mediated by PARP1, which can enhance the effectiveness of radiotherapy and chemotherapy.
Olaparib is used to treat various BRCA-related tumors, including ovarian cancer, breast cancer, pancreatic cancer, and prostate cancer. It received its first FDA and EU approvals in December 2014.
In a phase III study, Olaparib was used to treat metastatic castration-resistant prostate cancer (mCRPC) and its precursor, metastatic hormone-sensitive prostate cancer (mHSPC). These forms of late-stage prostate cancer have shown resistance to initial treatments such as surgery and hormone therapy and have spread beyond the prostate to other tissues.
The approval of Olaparib has been a significant advancement in cancer treatment, especially for patients with BRCA-related tumors and advanced prostate cancer. By targeting specific DNA repair pathways, Olaparib has demonstrated impressive results in clinical trials, providing new hope for patients with challenging forms of cancer and offering an effective treatment option where traditional therapies have shown limited success. Ongoing research continues to explore the potential of Olaparib in other cancer types and its use in combination therapies to further improve patient outcomes.
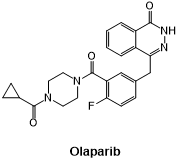
Fig 4. The structural formula of Olaparib.
Ruxolitinib:
It is an orally administered first-in-class Janus kinase (JAK) 1 and 2 inhibitor. It was initially approved by the FDA in 2011 for the treatment of adult patients with myelofibrosis, a rare and progressive blood cancer characterized by increased fibrous tissue in the bone marrow. In 2014, it was approved for the treatment of polycythemia vera (PV), another rare and slow-progressing blood cancer characterized by an increase in red blood cell production, although its pathogenesis remains poorly understood.
In 2019, the FDA approved Ruxolitinib for the treatment of steroid-refractory acute graft-versus-host disease (SR-aGVHD), a complication that can occur after stem cell transplantation.
Notably, Ruxolitinib has been investigated for its potential use in treating COVID-19 patients with severe systemic inflammation. In a phase II clinical trial, Ruxolitinib showed promise in improving outcomes for lymphopenic COVID-19 patients. However, subsequent phase III trials found that Ruxolitinib did not meet its primary endpoint of reducing the number of hospitalizations in severely ill COVID-19 patients with complications. As a result, the drug has not been approved for the treatment of COVID-19.
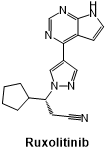
Fig 5. The structural formula of Ruxolitinib.
Venetoclax:
It is a breakthrough oral medication that has revolutionized the treatment of certain types of blood cancers. It is a selective inhibitor of B-cell lymphoma-2 (BCL-2), a protein that promotes the survival of cancer cells by preventing programmed cell death (apoptosis). By inhibiting BCL-2, Venetoclax promotes apoptosis in cancer cells, leading to their elimination and regression of the tumor.
Initially approved by the FDA in 2016, Venetoclax has shown remarkable efficacy in treating chronic lymphocytic leukemia (CLL), the most common form of adult leukemia, particularly in patients with the 17p deletion or TP53 mutation, which are associated with poor prognosis and limited treatment options. It has also been approved for the treatment of acute myeloid leukemia (AML) in patients with specific genetic abnormalities.
Venetoclax is often used in combination with other anti-cancer therapies to enhance its effectiveness. The drug’s ability to induce apoptosis in cancer cells has led to improved response rates and prolonged survival in many patients.
However, like all medications, Venetoclax may have side effects, and patients must be carefully monitored during treatment. Despite this, its approval represents a significant advancement in cancer therapy, offering renewed hope to patients with previously challenging-to-treat blood cancers. Ongoing research continues to explore the potential of Venetoclax in other hematologic malignancies and in combination with other therapies to further improve patient outcomes.
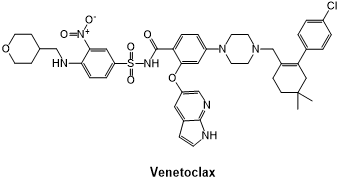
Fig 6. The structural formula of Venetoclax.
Acalabrutinib:
It is a promising oral medication that belongs to a class of drugs called Bruton’s tyrosine kinase (BTK) inhibitors. It has emerged as a revolutionary treatment for certain B-cell malignancies. Acalabrutinib works by selectively inhibiting the BTK enzyme, which plays a crucial role in the survival and proliferation of cancerous B-cells.
The drug received its first approval from the FDA in 2019 and has since shown impressive efficacy in treating various hematologic cancers, particularly chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL).
One of the key advantages of Acalabrutinib is its specificity, which allows for more targeted therapy and reduced off-target side effects compared to older BTK inhibitors. This results in a better safety profile and improved tolerability for patients.
Clinical trials have demonstrated that Acalabrutinib leads to significant responses in patients with relapsed or refractory CLL and MCL, even in those who have shown resistance to previous treatments. Additionally, it has also shown promising results in previously untreated patients, further establishing its role as a front-line therapy option.
As research continues, Acalabrutinib holds the potential to transform the treatment landscape for B-cell malignancies, offering new hope and improved outcomes for patients facing these challenging cancers.
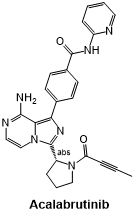
Fig 7. The structural formula of Acalabrutinib.
Tafamidis:
It is a groundbreaking medication used to treat a rare and progressive genetic disorder known as transthyretin amyloidosis (ATTR). ATTR is characterized by the abnormal accumulation of amyloid deposits in various tissues, leading to organ dysfunction and significant health issues. Tafamidis is specifically designed to stabilize the transthyretin (TTR) protein, preventing its misfolding and subsequent formation of amyloid deposits.
The drug was first approved by the FDA in 2018 for the treatment of hereditary ATTR amyloidosis with polyneuropathy, a form of the disease that primarily affects the peripheral nervous system. Tafamidis has shown remarkable efficacy in slowing disease progression and preserving nerve function, resulting in improved quality of life for patients.
In 2019, the FDA also approved Tafamidis for the treatment of ATTR cardiomyopathy, a form of the disease that affects the heart. By stabilizing TTR, Tafamidis can help mitigate the damaging effects of amyloid deposits on cardiac function, reducing the risk of heart-related complications and improving overall cardiac health.
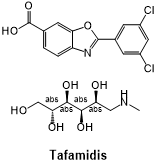
Fig 8. The structural formula of Tafamidis.
Tafamidis represents a significant advancement in the management of ATTR, offering hope and improved outcomes for patients living with this devastating and life-threatening condition. Ongoing research continues to explore its potential in other forms of amyloidosis and to further optimize its use in the treatment of ATTR.
Reference
Cameron, F., & Sanford, M. (2014). Ibrutinib: first global approval. Drugs, 74(2), 263–271.
Deeks E. D. (2015). Olaparib: first global approval. Drugs, 75(2), 231–240.
Deeks E. D. (2016). Venetoclax: First Global Approval. Drugs, 76(9), 979–987.