Advancing Cancer Therapy with Catalytic Topoisomerase II Inhibitors: A Safer Pathway Forward
Abstract
Topoisomerase II (Topo II) inhibitors, particularly non-DNA-damaging catalytic inhibitors, are emerging as promising alternatives in cancer therapy due to their potential for reducing adverse effects associated with traditional DNA-damaging agents. Catalytic inhibitors target the enzyme’s function without inducing DNA strand breaks, thereby offering a safer mechanism for targeting cancer cells. Current research focuses on enhancing specificity for the Topo IIα isoform, optimizing inhibitor design through molecular dynamics simulations, and exploring synergistic combinations with other therapeutic agents, such as histone deacetylase (HDAC) inhibitors. Additionally, the structural modification of natural compounds shows potential for developing new Topo II inhibitors that are both effective and biologically compatible. This review highlights the future directions and therapeutic implications of Topo II catalytic inhibitors, underscoring their role in advancing cancer treatment options.
Introduction to Topoisomerase II as an Anticancer Target
Topoisomerase II (Topo II) is a crucial enzyme responsible for managing DNA topology during cellular processes like replication and transcription. Its primary function is to introduce transient breaks in the DNA double helix, allowing the molecule to unwind and alleviate supercoiling or knotting that can occur during cell division. Because of this role, Topo II is indispensable for rapidly proliferating cells, such as cancer cells, making it an attractive target for anticancer therapies. Topo II exists in two isoforms in humans: Topo IIα, which is vital for cell proliferation, and Topo IIβ, which is more widely expressed but is not essential for cell survival.
Cancer research has shown significant interest in Topo II inhibitors due to their ability to disrupt DNA processes in cancer cells. These inhibitors are generally classified into two categories: Topo II poisons and catalytic inhibitors. Topo II poisons work by stabilizing the DNA-Topo II complex after DNA cleavage, preventing DNA repair and leading to cell death. While effective, these drugs, which include etoposide and doxorubicin, are associated with severe side effects, such as cardiotoxicity and secondary malignancies, because of their DNA-damaging effects.
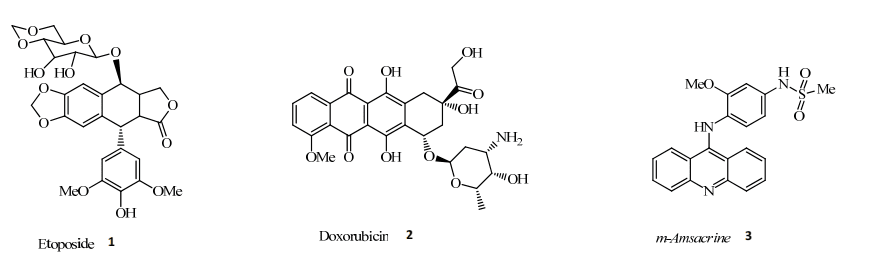
Figure 1. Structure of topoisomerase Ⅱ poisons
In contrast, Topo II catalytic inhibitors are emerging as safer alternatives, as they prevent enzyme function without inducing direct DNA breaks. Catalytic inhibitors work by interfering with enzyme processes such as ATP binding and hydrolysis or DNA binding. Recent studies suggest that catalytic inhibitors may offer an effective approach to suppress cancer cell proliferation while reducing the risk of adverse effects. This potential has led to a surge in research focused on discovering new Topo II catalytic inhibitors that could provide more targeted and safer cancer therapies.
Challenges with Current Topoisomerase II Poisons
Topoisomerase II (Topo II) poisons are widely used in chemotherapy due to their ability to disrupt DNA processes and induce cancer cell death. These compounds, such as etoposide and doxorubicin, stabilize the Topo II-DNA complex after it cleaves the DNA strands. By preventing the DNA from being resealed, they create double-strand breaks, ultimately leading to apoptosis in rapidly dividing cancer cells. While effective, Topo II poisons present substantial challenges that limit their clinical application, particularly in terms of safety and patient outcomes.
One of the main issues with Topo II poisons is their potential for off-target effects. Because these drugs induce DNA damage, they can harm normal, healthy cells in addition to cancer cells. This mechanism underlies many of their side effects, which include myelosuppression, nausea, and, in severe cases, cardiotoxicity. For example, doxorubicin is associated with a risk of cardiotoxicity that can lead to irreversible heart damage in some patients, especially at high doses or with prolonged treatment. Additionally, Topo II poisons have been linked to an increased risk of secondary malignancies, such as treatment-related leukemia, due to the DNA damage they inflict on non-cancerous cells.
Another challenge is the development of drug resistance in cancer cells treated with Topo II poisons. Cancer cells can develop mechanisms to evade the DNA-damaging effects of these drugs, such as upregulating drug efflux pumps or mutations that affect drug binding. This resistance reduces the efficacy of Topo II poisons over time, making it difficult to achieve long-term treatment success.
These limitations have driven interest in developing alternative Topo II inhibitors that avoid DNA damage while targeting the enzyme’s function. Catalytic inhibitors, for instance, offer a non-DNA-damaging approach, interfering with the enzyme’s catalytic cycle or ATP binding without creating breaks in the DNA. By sparing normal cells from DNA damage, these inhibitors may provide safer, more targeted cancer treatment options, which could address some of the most serious limitations of current Topo II poisons.
Emerging Catalytic Inhibitors and Their Mechanisms
Catalytic inhibitors of topoisomerase II (Topo II) are gaining interest in cancer research due to their potential to reduce side effects associated with DNA-damaging agents. Unlike traditional Topo II poisons, which stabilize the Topo II-DNA cleavage complex and cause double-strand DNA breaks, catalytic inhibitors prevent the enzyme’s function without inducing direct DNA damage. This mechanism provides a promising approach for treating cancer with a lower risk of off-target effects, such as cardiotoxicity and secondary malignancies.
Catalytic inhibitors act by interfering with various stages of the Topo II catalytic cycle. These stages include enzyme-DNA binding, ATP binding and hydrolysis, and the enzyme’s DNA-cleavage-religation activity. One of the most common mechanisms of catalytic inhibition is ATP-competitive binding, where inhibitors prevent the binding of ATP to the enzyme, which is essential for the enzyme’s catalytic cycle. For instance, recent studies have identified ATP-competitive inhibitors from virtual screening databases, showing potential as Topo IIα inhibitors with minimal off-target effects.
Some catalytic inhibitors are derived from natural compounds or are structural analogs of known inhibitors. Ellipticine, an alkaloid from the Ochrosia plant, has shown inhibitory effects on Topo II without inducing DNA damage, making it a suitable candidate for further modification and development as a catalytic inhibitor. Another example is garcinol, a polyisoprenylated benzophenone, which has been shown to inhibit Topo II activity by interacting with ATP binding. Additionally, perimidine derivatives have been designed to selectively inhibit Topo IIα, binding competitively at the ATP-binding site and preventing enzyme catalysis.
In silico methods, such as molecular docking and virtual high-throughput screening (VHTS), are invaluable in identifying new catalytic inhibitors. These computational approaches help pinpoint compounds with high binding affinity for the Topo II ATP site, enabling researchers to efficiently explore large chemical databases. By prioritizing non-DNA-damaging mechanisms, catalytic inhibitors of Topo II have the potential to overcome some of the challenges posed by traditional Topo II poisons, offering new hope for safer and more targeted anticancer therapies.
Potential of Natural Products and Structural Analogues as Topoisomerase II Inhibitors
Natural products and their derivatives have become a valuable source of potential topoisomerase II (Topo II) inhibitors, offering unique structural diversity and often lower toxicity compared to synthetic drugs. Researchers are increasingly exploring these compounds as alternatives to traditional Topo II poisons due to their ability to selectively target enzyme mechanisms without causing significant DNA damage. These compounds include plant-derived alkaloids, flavonoids, and other bioactive molecules that inhibit Topo II through catalytic mechanisms, thus providing a promising avenue for anticancer drug development.
One example is ellipticine, an alkaloid derived from the Ochrosia species, which has shown inhibitory activity against both Topo I and Topo II. Ellipticine and its derivatives can interfere with DNA-related processes by binding selectively to the enzyme without stabilizing the DNA-Topo II complex. Studies on ellipticine analogs have demonstrated their potential to induce apoptosis in cancer cells with lower off-target effects, sparking interest in further structural modifications to enhance efficacy and selectivity.
Another notable compound is garcinol, a benzophenone isolated from the Garcinia species. Garcinol is known for its anti-inflammatory and antioxidant properties, but recent studies indicate its potential as a Topo II inhibitor through an ATP-competitive mechanism. Garcinol binds to the enzyme’s ATP-binding domain, inhibiting its catalytic activity without promoting DNA cleavage. This selective interaction reduces the risk of side effects commonly associated with DNA-damaging agents, positioning garcinol as a promising lead for developing safer cancer therapies.
Additionally, flavonoids such as quercetin and luteolin have shown Topo II inhibition. These naturally occurring compounds exhibit mild toxicity and are capable of blocking the enzyme’s catalytic cycle. Their structural versatility and ability to interact with various cellular targets make them attractive candidates for anticancer drug development. Modifications of flavonoid structures have led to the synthesis of several analogs with enhanced Topo II inhibitory effects, underscoring the potential of these natural scaffolds.
The discovery of natural Topo II inhibitors and their synthetic analogs highlights a shift towards safer, more targeted treatments. By exploiting the diversity of plant-derived compounds, researchers aim to create new therapies with reduced side effects and improved efficacy.
Future Directions and Research Implications for Topoisomerase II Inhibitors
The continued development of topoisomerase II (Topo II) inhibitors, particularly non-DNA-damaging catalytic inhibitors, represents a promising direction for cancer therapeutics. While Topo II poisons have been widely used in chemotherapy, their adverse side effects, such as cardiotoxicity and risks of secondary cancers, have accelerated the search for safer alternatives. Catalytic inhibitors, which prevent Topo II activity without causing DNA strand breaks, are becoming an area of intense research focus as they offer a potentially safer pathway for targeting cancer cells.
One future direction in this field is the refinement of catalytic inhibitors to enhance their specificity for the Topo IIα isoform, which is predominantly expressed in rapidly dividing cells, including cancer cells. By targeting Topo IIα specifically, researchers hope to achieve more selective tumor inhibition while minimizing effects on healthy tissues. Additionally, advances in computational techniques, such as molecular dynamic (MD) simulations, allow researchers to model and predict the interactions of catalytic inhibitors with Topo II at the atomic level. This approach enables the design of inhibitors that better fit the enzyme’s active sites, potentially improving efficacy and reducing off-target interactions.
Another promising area involves the combination of catalytic Topo II inhibitors with other therapeutic agents. For example, dual-action compounds that inhibit both Topo II and other key cellular targets, such as histone deacetylase (HDAC), are currently being explored for their synergistic anticancer effects. Such combinations could result in enhanced tumor cell death while lowering the dosage of each drug, which could mitigate side effects.
The structural modification of natural products also offers exciting potential for developing Topo II inhibitors. Many naturally derived compounds, including flavonoids and alkaloids, have shown initial Topo II inhibitory effects, and further modification can optimize these molecules for greater therapeutic value. This approach capitalizes on the structural diversity and biological compatibility of natural compounds, potentially leading to more effective and safer cancer therapies.
In sum, the future of Topo II inhibitor research lies in designing more selective, multi-targeted, and biologically compatible compounds. These advances could transform cancer treatment by providing safer, more effective options for patients.
References
- Wang, J. C. (2002). Cellular roles of DNA topoisomerases: A molecular perspective. Nature Reviews Molecular Cell Biology, 3(6), 430–440.
- Bollimpelli, V. S., Dholaniya, P. S., & Kondapi, A. K. (2017). Topoisomerase IIβ and its role in different biological contexts. Archives of Biochemistry and Biophysics, 633, 78–84.
- Skok, Ž., Durcik, M., & Kikelj, D. (2020). Discovery of new ATP-competitive inhibitors of human DNA topoisomerase IIα through screening of bacterial topoisomerase inhibitors. Bioorganic Chemistry, 102, 104049.
- Lee, C. J., Kang, J. S., & Lee, M. S. (2004). The study of doxorubicin and its complex with DNA by SERS and UV-resonance Raman spectroscopy. Bulletin of the Korean Chemical Society, 25(8), 1211–1216.
- Tosa, H., Iinuma, M., & Tsutsui, K. (1997). Inhibitory activity of xanthone derivatives isolated from some guttiferaeous plants against DNA topoisomerases I and II. Chemical and Pharmaceutical Bulletin, 45(3), 418–420.
- Mai, Y. W., Liang, C. C., & Huang, S. L. (2021). 9-Bromo-2,3-diethylbenzo[de]chromene-7,8-dione: A novel non-intercalative topoisomerase II catalytic inhibitor. Bioorganic Chemistry, 114, 105097.
- Di Micco, S., Masullo, M., & Bifulco, G. (2019). Garcinol and related polyisoprenylated benzophenones as topoisomerase II inhibitors: Biochemical and molecular modeling studies. Journal of Natural Products, 82(10), 2768–2779.
- Catanzaro, E., Arencibia, J. M., & Santini, A. (2020). Targeting topoisomerase II with trypthantrin derivatives: Discovery of antiproliferative agents to treat cancer. European Journal of Medicinal Chemistry, 202, 112504.
- Okoro, C. O., & Fatoki, T. H. (2023). A mini review of novel topoisomerase II inhibitors as future anticancer agents. International Journal of Molecular Sciences, 24(3), 2532.




