Advancing Cancer Treatment: The Future of Nucleoside Analogues in Precision Medicine
Abstract
Nucleoside and nucleobase analogues are integral to cancer therapy, offering potent cytotoxic effects through the disruption of DNA and RNA synthesis. Despite their efficacy, resistance mechanisms at the pharmacokinetic, pharmacodynamic, and tumor-specific levels limit their clinical potential. This article explores innovative strategies to overcome resistance, including the development of ProTides, enzyme inhibitors, and combination therapies. The role of precision medicine in tailoring treatments through biomarker-driven approaches and genomic profiling is emphasized, highlighting the integration of these advancements into clinical practice. Future perspectives focus on the promise of SAMHD1 inhibitors, personalized dosing regimens, and synthetic lethality to enhance therapeutic outcomes and re-sensitize resistant tumors. These advancements underscore the potential of nucleoside analogues to remain pivotal in precision oncology, addressing challenges in resistant and refractory cancers.
Introduction: The Promise and Challenge of Nucleoside Analogues
Nucleoside and nucleobase analogues represent a cornerstone in modern oncology, offering groundbreaking therapeutic avenues for both solid and hematological malignancies. As a subclass of antimetabolites, these agents mimic natural nucleosides, disrupting DNA and RNA synthesis and impairing nucleotide metabolism. Their cytotoxicity stems from their ability to interfere with cellular replication, targeting rapidly dividing cancer cells and inducing apoptosis. Initially developed for pediatric oncology, these compounds have since demonstrated their efficacy across diverse cancer types, including acute leukemias, pancreatic cancer, and colorectal cancer.
Despite their significant clinical utility, the challenge of resistance to nucleoside analogues remains a critical obstacle in cancer therapy. Resistance mechanisms arise at multiple levels, from pharmacokinetics—where drug delivery and stability are hindered—to pharmacodynamics, where intracellular activation and target engagement fail to elicit therapeutic effects. These multifaceted resistance pathways often diminish the efficacy of treatment, particularly in cases of aggressive or relapsed cancers.
In addition to pharmacokinetic and pharmacodynamic barriers, tumor-specific factors also play a critical role in resistance. Variations in transporter protein expression, such as equilibrative nucleoside transporters (hENTs), can significantly influence cellular drug uptake. Similarly, enzymatic factors, including cytidine deaminase and ribonucleotide reductase, dictate the intracellular activation or degradation of these analogues, further complicating their therapeutic potential. Understanding these complexities is essential for developing strategies to overcome resistance and optimize treatment outcomes.
Nucleoside analogues are not only crucial for first-line treatments but also hold potential in combination therapies, complementing other modalities like immunotherapy and radiation. Their success in such combinations underlines the need for innovative solutions to address resistance mechanisms. By leveraging advancements in molecular biology and pharmacology, the next generation of nucleoside analogues can offer renewed hope for patients with refractory cancers.
In this series, we will explore the intricate mechanisms of resistance, strategies for re-sensitizing tumors, and the role of precision medicine in optimizing nucleoside analogue therapies.
Understanding Resistance Mechanisms
The therapeutic potential of nucleoside and nucleobase analogues in oncology is often curtailed by resistance mechanisms that arise at multiple levels, posing significant challenges to effective cancer treatment. Resistance can be broadly classified into pharmacokinetic, pharmacodynamic, and tumor-specific mechanisms, each of which hinders the drug’s ability to exert its cytotoxic effects.
At the pharmacokinetic level, resistance begins with the inability of nucleoside analogues to reach their target sites. The blood-brain barrier (BBB), for instance, limits the delivery of many hydrophilic analogues to central nervous system malignancies. Moreover, enzymatic degradation by cytidine deaminase or dihydropyrimidine dehydrogenase in the plasma reduces the stability and bioavailability of these compounds, rendering them less effective before they even reach the tumor cells.
Pharmacodynamic resistance involves failures at the cellular level, including reduced drug uptake, insufficient activation, and inadequate target engagement. Cellular transporters, such as the human equilibrative nucleoside transporters (hENTs) and human concentrative nucleoside transporters (hCNTs), are pivotal for drug entry into cells. A decrease in transporter expression can significantly impair drug efficacy. Intracellularly, enzymes like deoxycytidine kinase (dCK) play a crucial role in converting nucleoside analogues into their active forms. Downregulation of dCK or mutations in related pathways leads to inadequate drug activation, further contributing to resistance.
Tumor-specific factors also complicate the landscape of resistance. Variations in the tumor microenvironment, genetic mutations, and the overexpression of certain enzymes like ribonucleotide reductase and SAMHD1 can mitigate the effectiveness of nucleoside analogues. These factors collectively allow cancer cells to evade cytotoxicity, even in the presence of otherwise potent drugs.
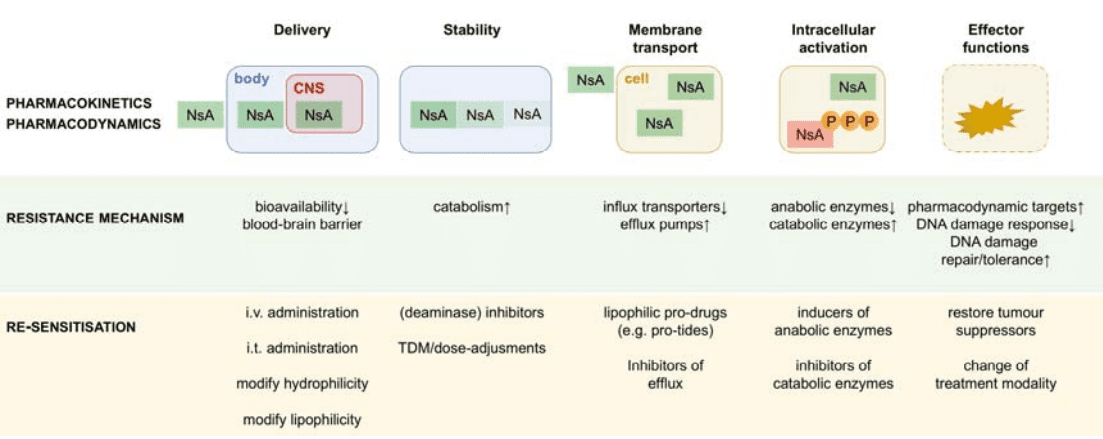
Figure 1. Schematic representation of the levels of resistance to nucleobase/nucleoside analogues.
Overcoming these barriers requires a comprehensive understanding of the underlying mechanisms. Strategies such as transporter modulation, enzyme inhibition, and the development of lipophilic prodrugs have shown promise in preclinical and clinical settings. Addressing these resistance pathways is critical to maximizing the therapeutic efficacy of nucleoside analogues and improving outcomes for patients with resistant cancers.
Innovative Strategies for Re-Sensitisation
Addressing resistance to nucleoside and nucleobase analogues requires innovative approaches to re-sensitize tumors and enhance the efficacy of these critical antimetabolite drugs. Advances in pharmacology and molecular biology have paved the way for strategies targeting the key mechanisms that underlie resistance, from pharmacokinetics to intracellular processes.
One promising avenue lies in the use of enzyme inhibitors to counteract resistance caused by enzymatic degradation. Cytidine deaminase (CDA) and dihydropyrimidine dehydrogenase (DPD) are known to metabolize nucleoside analogues into inactive forms, reducing their efficacy. Inhibitors such as tetrahydrouridine and eniluracil have been developed to block these enzymes, increasing drug stability and bioavailability in patients with high enzymatic activity. Similarly, SAMHD1 inhibitors are being explored to enhance the intracellular retention of active drug metabolites, preventing premature deactivation.
Transporter modulation is another key area of focus. Downregulation of nucleoside transporters like hENT1 and hCNT3 significantly reduces drug uptake into tumor cells. To address this, researchers are investigating approaches such as prodrug formulations that bypass reliance on these transporters. Prodrugs like elacytarabine have shown potential by incorporating lipophilic modifications to improve cellular uptake.
For resistance linked to inadequate drug activation, phosphoramidate prodrug technology (e.g., ProTides) has been transformative. By bypassing the need for enzymatic activation by deoxycytidine kinase (dCK), ProTides deliver the active monophosphate form of the drug directly into cells. This strategy has shown promise in preclinical and clinical trials, particularly for drugs like gemcitabine and cytarabine.
In addition, combination therapies are being employed to synergize the effects of nucleoside analogues with other agents. For instance, pairing nucleoside analogues with MEK-ERK pathway inhibitors can downregulate ribonucleotide reductase, reducing competition from endogenous nucleotides and enhancing drug efficacy.
These innovative strategies not only hold the potential to overcome resistance but also pave the way for personalized medicine, tailoring treatments to the unique resistance profiles of individual tumors.
The Role of Precision Medicine
Precision medicine is revolutionizing oncology by tailoring treatments to the genetic, molecular, and phenotypic profiles of individual patients and their tumors. For nucleoside and nucleobase analogues, this approach is particularly transformative, addressing the heterogeneity of resistance mechanisms that often limit their efficacy.
One of the foundational principles of precision medicine is the identification of biomarkers that predict drug response. In the case of nucleoside analogues, biomarkers such as the expression levels of human equilibrative nucleoside transporters (hENTs) and deoxycytidine kinase (dCK) can guide treatment decisions. For instance, low hENT1 expression in pancreatic cancer has been associated with poor response to gemcitabine. By incorporating such biomarkers into clinical workflows, clinicians can identify patients who are most likely to benefit from these therapies or consider alternative treatments for those with resistant profiles.
Genomic profiling has also become a cornerstone of precision medicine. Mutations and polymorphisms in genes encoding key enzymes, such as cytidine deaminase (CDA) and SAMHD1, influence the metabolism and efficacy of nucleoside analogues. For example, polymorphisms in CDA can lead to reduced enzymatic activity, resulting in higher drug toxicity. Tailoring drug dosages or combining nucleoside analogues with CDA inhibitors can optimize outcomes for such patients.
Additionally, precision medicine enables the development of individualized combination therapies. For tumors with elevated ribonucleotide reductase (RNR) expression, pairing nucleoside analogues with RNR inhibitors can enhance therapeutic efficacy. Similarly, targeting SAMHD1 with small-molecule inhibitors can increase intracellular drug retention, overcoming one of the key barriers to treatment success.
Advancements in pharmacogenomics are further enabling personalized dosing regimens, minimizing toxicity while maintaining efficacy. Therapeutic drug monitoring (TDM) has been successfully employed in some cases to adjust treatment based on real-time pharmacokinetic data, ensuring optimal drug exposure.
Through biomarker-driven treatment selection, combination therapies, and individualized dosing strategies, precision medicine is poised to redefine the role of nucleoside analogues in oncology, offering hope for improved patient outcomes and overcoming resistance.
Future Perspectives and Clinical Implications
The future of nucleoside and nucleobase analogues in cancer therapy lies in the integration of innovative strategies and personalized approaches to overcome resistance and enhance therapeutic outcomes. As understanding of the mechanisms driving resistance evolves, novel therapeutic strategies are emerging to ensure these drugs maintain their pivotal role in oncology.
One promising avenue is the development of next-generation nucleoside analogues designed to evade common resistance mechanisms. Advances in prodrug technology, such as phosphoramidate prodrugs (ProTides), allow efficient intracellular delivery of active drug metabolites, bypassing reliance on cellular transporters or activation enzymes. Drugs like NUC-1031, a ProTide derivative of gemcitabine, have shown efficacy in overcoming resistance in clinical trials.
Combination therapies are another key focus. Targeting complementary pathways, such as pairing nucleoside analogues with ribonucleotide reductase (RNR) inhibitors, can enhance cytotoxicity by reducing the availability of competing endogenous nucleotides. Similarly, combining these analogues with MEK-ERK pathway inhibitors or immune checkpoint blockade may improve outcomes in resistant cancers.
Biomarker-driven therapy selection will play a critical role in optimizing treatment. Genomic profiling of tumors to assess transporter expression, enzymatic activity, and mutations will guide the selection of nucleoside analogues and their combinations, ensuring efficacy while minimizing toxicity. Tools like therapeutic drug monitoring (TDM) and pharmacogenomic testing can further refine dosing strategies.
The development of SAMHD1 inhibitors represents a significant breakthrough in addressing intracellular resistance. By preventing the degradation of active drug metabolites, these inhibitors hold the potential to re-sensitize resistant tumors. Additionally, novel approaches targeting DNA repair mechanisms, such as PARP inhibitors, can create synthetic lethality when combined with nucleoside analogues.
As research progresses, the clinical implications of these advancements will reshape the oncology landscape. By integrating molecular insights with innovative drug development, nucleoside and nucleobase analogues are poised to maintain their relevance in precision oncology, offering renewed hope for patients with resistant or refractory cancers.
References
- Tsesmetzis, N., Paulin, C. B. J., Rudd, S. G., & Herold, N. (2018). Nucleobase and nucleoside analogues: Resistance and re-sensitisation at the level of pharmacokinetics, pharmacodynamics and metabolism. Cancers, 10(7), 240. DOI
- Jordheim, L. P., Durantel, D., Zoulim, F., & Dumontet, C. (2013). Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nature Reviews Drug Discovery, 12(6), 447–464. DOI
- Galmarini, C. M., Thomas, X., & Dumontet, C. (2002). Resistance to cytotoxic nucleoside analogues in cancer therapy. Biochimica et Biophysica Acta (BBA) – Reviews on Cancer, 1587(2–3), 194–206. DOI
- Slusarczyk, M., Lopez, M. H., Balzarini, J., & McGuigan, C. (2014). Application of ProTide technology to gemcitabine: A successful approach to overcome the key cancer resistance mechanisms leads to a new agent (NUC-1031) in clinical development. Journal of Medicinal Chemistry, 57(4), 1531–1542. DOI
- Greenhalf, W., Ghaneh, P., Neoptolemos, J. P., & Palmer, D. H. (2014). Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. Journal of the National Cancer Institute, 106(1), djt347. DOI
- Hagenkort, A., Paulin, C. B. J., Desroses, M., & Helleday, T. (2017). dUTPase inhibition augments replication defects of 5-Fluorouracil. Oncotarget, 8(15), 23713–23726. DOI
- McGuigan, C., Cahard, D., & Balzarini, J. (2009). Aryloxy phosphoramidate triesters: A technology for delivering monophosphorylated nucleosides and sugars into cells. ChemMedChem, 4(12), 1779–1791. DOI
- Bergman, A. M., Peters, G. J., & Lankelma, J. (2005). Ribonucleotide reductase in the resistance of solid tumors to gemcitabine. Advances in Enzyme Regulation, 45(1), 118–135. DOI
- Mehellou, Y., & Rattan, H. S. (2015). ProTide technology: From the concept to the clinic. Journal of Medicinal Chemistry, 58(6), 2293–2302. DOI




