Advancing the Fight Against Fungal Infections: Immune Mechanisms, Diagnostic Challenges, and Emerging Therapeutic Strategies
Abstract
Fungal infections, especially those caused by opportunistic pathogens such as Candida, Aspergillus, and Cryptococcus, present significant challenges for diagnosis and treatment, especially for immunocompromised individuals. The complex response of the immune system to fungal pathogens involves both innate and adaptive mechanisms, with key immune cells, including macrophages, neutrophils, and helper T cells, playing a critical role in pathogen recognition and clearance. Despite advances in the understanding of immune mechanisms, the diagnosis of fungal infections remains difficult due to the diversity of fungal species and the lack of rapid diagnostic tools. Current treatment options, including azoles, echinocandins, and polyenes, face limitations such as toxicity, resistance, and narrow-spectrum activity. New therapeutic strategies, including novel antifungal agents, immunotherapies, and vaccines, are being developed to overcome these challenges. The future of fungal infection management lies in improved diagnostic techniques, expanded access to antifungal treatments, and enhanced immune responses through targeted interventions. This review discusses the immune mechanisms of fungal infections, the challenges of diagnosis and treatment, and the potential for new therapeutic approaches to improve patient outcomes.
Introduction to Human Fungal Pathogens and Immune Responses
Fungal infections in humans are a significant global health concern, with diverse pathogens affecting both immunocompromised and healthy individuals. Human fungal pathogens, such as Candida, Aspergillus, Cryptococcus, and Histoplasma, are the culprits behind a variety of infections that can range from superficial skin diseases to life-threatening systemic infections. These pathogens are often opportunistic, taking advantage of compromised immune systems, which is why individuals with conditions like HIV/AIDS, cancer, diabetes, and organ transplants are at a higher risk. The immune system plays a crucial role in defending against fungal infections. The innate immune system, which provides the first line of defense, detects fungal pathogens through pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and C-type lectin receptors (CLRs). These receptors recognize fungal cell wall components like chitin and β-glucan, triggering an inflammatory response to contain the infection. In addition, phagocytic cells, such as macrophages and neutrophils, are essential for clearing fungal pathogens by engulfing and destroying them.
While innate immunity provides early defense, the adaptive immune system is also key to controlling fungal infections. T-helper cells (Th1 and Th17) play critical roles in the activation of macrophages and the recruitment of neutrophils to the site of infection. Furthermore, the production of antibodies by B-cells helps neutralize certain fungal pathogens, aiding in their clearance.
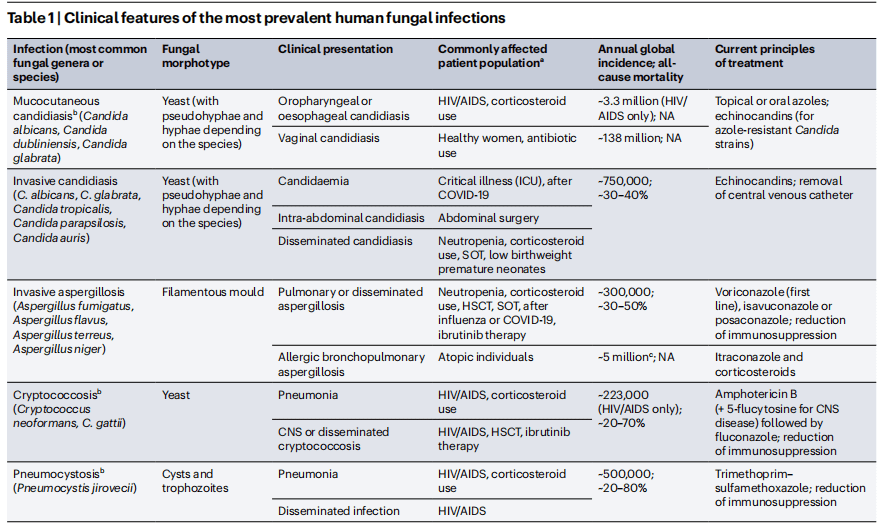
However, the immune response to fungal infections is often insufficient, especially in immunocompromised individuals. Fungal pathogens have evolved mechanisms to evade immune detection, making these infections difficult to treat. Understanding the immune mechanisms involved in fungal infections is vital for developing novel therapeutic strategies to improve outcomes and combat the rising challenge of antifungal resistance.
Immune Mechanisms in Fungal Infections
The immune response to fungal infections is a complex interplay between the host’s immune system and the invading fungal pathogens. Both the innate and adaptive arms of the immune system are engaged in recognizing, containing, and eliminating fungal infections, yet these mechanisms are often evaded by pathogenic fungi through various survival strategies.
At the forefront of the immune response are innate immune cells, particularly macrophages, neutrophils, and dendritic cells. These cells are critical in detecting fungal pathogens early through pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), C-type lectin receptors (CLRs), and NOD-like receptors (NLRs). These receptors recognize conserved molecular patterns found on the fungal cell wall, such as β-glucans, chitin, and mannan. Upon recognition, PRRs initiate a cascade of signals that promote phagocytosis, cytokine production, and inflammation, which are essential for controlling fungal growth and dissemination.
Neutrophils, as one of the first responders to fungal infections, play a pivotal role in fungal clearance. They engage in phagocytosis and release reactive oxygen species (ROS) and neutrophil extracellular traps (NETs) to kill the fungus. However, many fungal pathogens have evolved mechanisms to resist these killing strategies. For example, Aspergillus and Candida species can neutralize reactive oxygen species, making them more resilient to neutrophil-mediated killing.
The adaptive immune response is equally important in managing fungal infections, particularly through the action of T-helper (Th) cells. Th1 and Th17 cells, in particular, are critical for coordinating the immune response by stimulating macrophages and enhancing neutrophil recruitment. Th17 cells also produce interleukin-17 (IL-17), which promotes the recruitment of neutrophils to the site of infection and supports fungal clearance.
Despite these immune defenses, the ability of fungi to form biofilms and their ability to adapt to host environments contribute to persistent and chronic infections, especially in immunocompromised individuals. Understanding these immune mechanisms is crucial for the development of more effective antifungal therapies and immunotherapies.
Challenges in Diagnosing and Treating Fungal Infections
Fungal infections present significant challenges in both diagnosis and treatment, particularly in immunocompromised patients where these infections are often severe and difficult to manage. Fungal pathogens are diverse, with over 300 species capable of causing human disease, making accurate and timely diagnosis a complex task. One of the primary hurdles in diagnosing fungal infections is the lack of reliable, rapid diagnostic tests that can distinguish between different types of fungal pathogens and differentiate between colonization and infection. Current diagnostic methods, including culture-based techniques, microscopy, and histopathology, can be time-consuming and may not provide accurate results in the early stages of infection.
Molecular diagnostic tools, such as PCR and DNA sequencing, offer promising improvements in speed and sensitivity, but they remain limited by high costs and accessibility. Furthermore, the broad range of fungal species that can cause infections, coupled with the fact that many fungi are opportunistic, complicates the identification of the pathogen and its associated resistance profiles. As a result, early detection often occurs too late, by which time the infection may have already become systemic or resistant to treatment.
Treatment of fungal infections is equally challenging due to the limited number of antifungal agents available and the emergence of antifungal resistance. The most commonly used antifungal drugs include azoles, echinocandins, and polyenes, but each class of drugs has its own limitations. Azoles, for example, face significant resistance from pathogens like Candida and Aspergillus species, making treatment options even more limited. Moreover, these antifungals often cause side effects such as liver toxicity, which may further restrict their use, especially in immunocompromised patients. Newer antifungal agents and drug combinations are under development, but there remains a clear need for more effective therapies. Addressing the diagnostic and therapeutic challenges requires better surveillance, faster diagnostic tests, and the development of novel antifungal drugs with fewer side effects and broader spectrum efficacy.
Therapeutic Advances and New Strategies
In recent years, there has been substantial progress in the development of new therapeutic strategies for fungal infections, addressing the critical need for more effective treatments. Current antifungal therapies, which primarily include azoles, polyenes, and echinocandins, have limitations such as toxicity, narrow spectrum, and rising antifungal resistance. Consequently, researchers are exploring novel approaches to overcome these challenges.One promising direction is the development of new antifungal agents with novel mechanisms of action. For instance, the introduction of antifungal drugs that inhibit fungal cell wall biosynthesis, such as the echinocandins, has revolutionized treatment, particularly for Candida infections. However, resistance to these agents has emerged, prompting further exploration of alternative strategies, such as drugs that target fungal metabolism or virulence factors.
Immunotherapy is another exciting frontier. Given the pivotal role of the immune system in controlling fungal infections, enhancing host immunity through targeted immune-modulation could provide an effective adjunct to traditional antifungal drugs. Monoclonal antibodies targeting fungal virulence factors or immune checkpoint inhibitors to enhance the immune response are being investigated in clinical trials.Additionally, vaccines against common fungal pathogens, such as Candida and Aspergillus, are being actively developed. Although no fungal vaccine is yet approved for clinical use, recent studies have shown promising results in animal models, highlighting the potential for preventative therapy in at-risk populations.
Lastly, the rise of combination therapies, which use multiple drugs to target different fungal components or immune pathways, offers a promising strategy for combating resistance and improving patient outcomes. These combinations are designed to reduce the risk of resistance while providing synergistic effects that enhance antifungal activity.
Conclusion and Future Directions
Opportunistic fungal infections cause a substantial disease burden globally and are a major threat to the well-being of vulnerable patients. The past decade of research on fungal immunology has moved at a remarkable pace and has yielded exciting advances in our basic understanding of the cellular and molecular determinants of antifungal defences. In this Review, we have delineated promising immunogenomic factors that may enable personalized risk assessment and prognosis of patients at risk of fungal disease, and adjunctive immunotherapeutic strategies that may protect fungus-infected patients by modulating antifungal responses. We have highlighted the expanding universe of inherited and iatrogenic immunodeficiency conditions that predispose to opportunistic fungal disease, as well as the conceptual advances in our understanding of protective and pathogenic antifungal immune pathways that have been derived from studying these conditions. We have also discussed how the endogenous mycobiota shape immune homeostasis and regulate responses to neoplasia, infections, autoimmunity and other exogenous insults. Moving forward, further increasing our understanding of tissue-specific mechanisms of antifungal defence should help to improve the management of lifethreatening fungal infections and curtail their detrimental impact on human health.
References
- Lionakis, M. S., Drummond, R. A., & Hohl, T. M. (2022). Immune responses to human fungal pathogens and therapeutic prospects. Nature Reviews Immunology.
- Brown, G. D., Denning, D. W., & Gow, N. A. R. (2012). Fungal infections and the immune system: Pathophysiology and treatment strategies. Clinical Microbiology Reviews, 25(4), 616-634.
- Casadevall, A., & Pirofski, L. A. (2007). The damage-response framework of microbial pathogenesis. Nature Reviews Microbiology, 5(3), 182-189.
- Wang, J., & Zha, J. (2015). Neutrophils and the immune response to fungal infections. Frontiers in Immunology, 6, 249.
- Lenardon, M. D., & Ramage, G. (2014). Immunity and host defense mechanisms to fungal infections. Fungal Biology Reviews, 28(1), 47-59.
- Romani, L. (2011). Immunity to fungal infections. Nature Reviews Immunology, 11(4), 275-288.
- Gresnigt, M. S., & Netea, M. G. (2013). The innate immune response to fungal infections: What can we learn from the host? Immunological Reviews, 255(1), 28-44.
- Gresnigt, M. S., & Netea, M. G. (2013). The innate immune response to fungal infections: What can we learn from the host? Immunological Reviews, 255(1), 28-44.
- Kauffman, C. A. (2018). Invasive fungal infections: Diagnosis and treatment. Clinical Infectious Diseases, 66(9), 1413-1421.
- Sheehan, D. J., & Hitchcock, C. A. (2010). Antifungal drug resistance: Mechanisms and clinical consequences. Clinical Infectious Diseases, 51(3), 307-315.
- Groll, A. H., & Rapp, H. (2011). Fungal infections in the immunocompromised host: New treatment strategies. Infectious Disease Clinics of North America, 25(4), 617-634.




