Apixaban: A Breakthrough in Anticoagulation Therapy and Stroke Prevention
Abstract
Apixaban, a selective Factor Xa inhibitor and direct oral anticoagulant (DOAC), has greatly enhanced the treatment of thromboembolic illnesses, including deep vein thrombosis (DVT), pulmonary embolism (PE), and stroke prevention in nonvalvular atrial fibrillation. This blog post highlights the benefits of apixaban over conventional anticoagulants like warfarin by examining its mechanism of action, clinical uses, pharmacokinetics, and continuing research. Presently, studies are concentrated on improving the use of reversal medicines and broadening its therapeutic applications, especially in combination therapies and cancer-associated thrombosis. There are encouraging opportunities to improve patient outcomes in anticoagulant therapy with Apixaban’s possible additional indications and future use in personalized medicine.
Introduction
Mainly used for the prevention and treatment of thromboembolic disorders, such as stroke in patients with nonvalvular atrial fibrillation (AF) and the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), Apixaban, also marketed under the trade name Eliquis, is a leading direct oral anticoagulant (DOAC) that has revolutionized the field of anticoagulation therapy. Apixaban’s rise in the medical field is attributed to its efficacy, safety profile, and convenience, which make it a superior option to conventional anticoagulants like warfarin.
Apixaban is one of the most well-known direct Factor Xa inhibitors, and its development has been one of the most important advances in anticoagulant therapy. There is less need for frequent blood testing with Apixaban since it has a more predictable pharmacokinetic and pharmacodynamic profile than warfarin, which necessitates frequent monitoring and dose modifications. Because of its predictability and decreased risk of serious bleeding, especially cerebral hemorrhage, Apixaban has revolutionized anticoagulant management.
Apixaban’s significance goes beyond its medicinal uses. Given that several guidelines now propose using DOACs as first-line therapy for both the management of venous thromboembolism (VTE) and stroke prevention in atrial fibrillation (AF), their development marks a substantial shift toward their use. Furthermore, Apixaban’s status as a mainstay of anticoagulant therapy has been reinforced by its consistent dosage schedule and low levels of food and medication interactions.
Research and clinical trials are anticipated to broaden the indications of Apixaban and enhance its utilization in diverse patient demographics as the medical community endeavors to fully realize its potential.
Mechanism of Action
Factor Xa is an important enzyme in the coagulation cascade that converts prothrombin to thrombin. Apixaban is a strong and selective inhibitor of Factor Xa. After then, fibrinogen is changed into fibrin by thrombin, which causes a blood clot to develop. Apixaban efficiently breaks this cascade by directly inhibiting Factor Xa, which lowers the production of thrombin and the consequent formation of clots. Because of its mode of action, Apixaban is very successful in preventing thromboembolic events in a number of clinical contexts, such as pulmonary embolism, deep vein thrombosis, and atrial fibrillation.
Apixaban’s ability to selectively target Factor Xa sets it apart from other anticoagulants like warfarin. Although warfarin works by preventing the manufacture of many coagulation factors that are dependent on vitamin K, such as Factor Xa, Apixaban’s direct inhibition provides a more focused method. This specificity lowers the possibility of bleeding, especially cerebral hemorrhage, which is a dangerous side effect of anticoagulant medication.
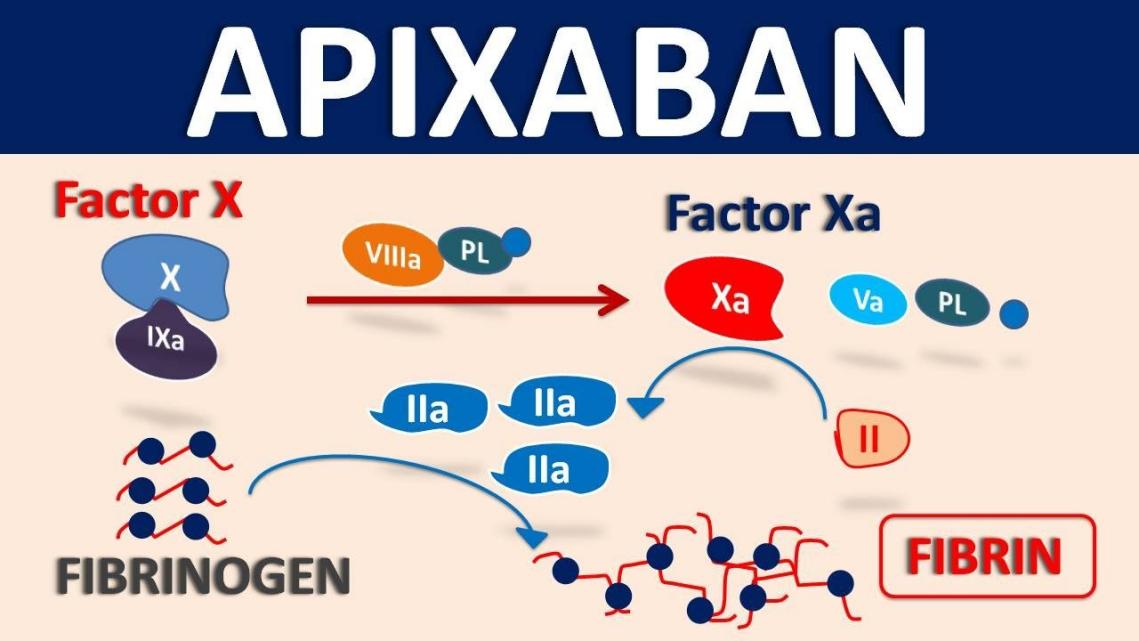
Fig.1 Apixaban mechanism of action
The quick start and stop of Apixaban’s mechanism of action is another benefit. After oral administration, apixaban achieves peak plasma concentrations in 3–4 hours, and its effects start to wear off in 12 hours. This makes it easier to monitor anticoagulation therapy and enables a more predictable anticoagulant response, particularly in circumstances requiring quick reversal, like emergency surgery or severe bleeding events.
In conclusion, the direct Factor Xa inhibitor mechanism of action of apixaban provides a strong, reliable, and regulated form of anticoagulation. Because of its good safety record and ability to prevent thromboembolic events, apixaban is now a mainstay of contemporary anticoagulant therapy.
Clinical Applications
Because of its effectiveness and safety record, apixaban has established itself as a mainstay in the management of thromboembolic diseases. For individuals with nonvalvular atrial fibrillation (AF), preventing strokes is one of the main clinical uses of apixaban. Due to the possibility of blood clots forming in the heart and migrating to the brain, patients with atrial fibrillation are more vulnerable to stroke. In these patients, apixaban dramatically lowers the risk of stroke without requiring routine blood monitoring—a requirement that is frequently associated with previous anticoagulants like warfarin.
Apixaban is licensed for the treatment and prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE), in addition to the prevention of stroke. Blood clots in the deep veins, generally in the legs, cause DVT, but clots that reach the lungs can cause PE, a potentially fatal illness. Apixaban is a great option for the short- and long-term therapy of various illnesses due to its consistent anticoagulant impact.
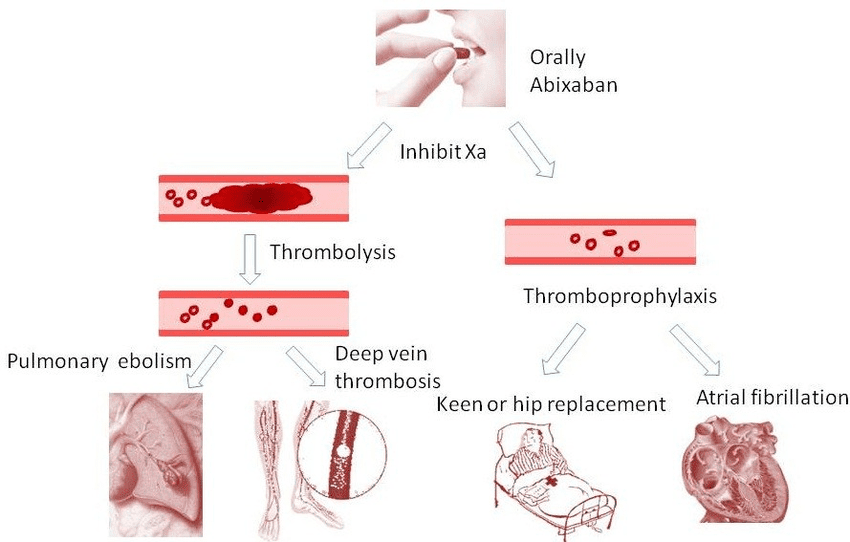
Fig. 2 Clinical application of apixaban
Clinical trials supporting Apixaban’s safety and efficacy in preventing venous thromboembolism (VTE) in surgical patients underpin its use in this context. Apixaban is also used in post-surgical prophylaxis, particularly after hip and knee replacement surgeries, where patients are at high risk of developing blood clots due to prolonged immobility. Apixaban provides effective clot prevention with a lower risk of bleeding compared to older anticoagulants.
Overall, apixaban is a major component of anticoagulant therapy and provides notable advantages over conventional therapies due to its wide range of clinical uses in the prevention and treatment of thromboembolic illnesses.
Advantages and Limitations
Apixaban is a popular option in many clinical contexts due to its various advantages over conventional anticoagulants such as warfarin. The absence of regular blood monitoring is one of the biggest benefits. As opposed to warfarin, which necessitates routine INR (International Normalized Ratio) measurements to guarantee the appropriate dosage, apaxaban has a consistent dose-response relationship, making management easier for patients and medical professionals.
Apixaban’s decreased risk of significant bleeding, including cerebral hemorrhage—a serious and frequently fatal side effect of anticoagulant therapy—is another important benefit. Apixaban has a better safety profile than warfarin, according to clinical trials, which makes it a safer choice for long-term treatment in a variety of individuals.
Apixaban is not without its drawbacks, either. Its cost, which is more than that of warfarin and may prevent certain patients from receiving it, is one of the main issues. Furthermore, even though apixaban has fewer food and medication interactions than warfarin, it is still not recommended for use in patients who have severe renal impairment or who are taking potent CYP3A4 and P-glycoprotein inhibitors. There is currently no widely available antidote for Apixaban, making it difficult to manage bleeding issues in patients taking the medication; nevertheless, certain reversal medicines have been developed and are in use.
Notwithstanding these drawbacks, many patients needing anticoagulant medication may find Apixaban to be a desirable alternative due to its benefits, especially in terms of safety and ease of use. As more information becomes available and healthcare systems adjust to include these newer anticoagulants in routine care, its use is probably going to keep growing.
Pharmacokinetics and Metabolism
Because of its two paths of elimination, quick absorption, and moderate bioavailability, apixaban has a pharmacokinetic and metabolic profile that makes it a dependable and predictable anticoagulant. Within three to four hours following oral dosing, Apixaban reaches peak plasma concentrations. Its around 50% bioavailability means that dose schedules can be easily adjusted, as food intake has little effect on it.
With a volume of distribution of about 21 liters, Apixaban is widely available. Its strong protein binding, especially to albumin, affects how it behaves pharmacokinetically. With minimal contributions from CYP3A5 and other routes, the cytochrome P450 enzyme CYP3A4 is principally responsible for the liver’s metabolism of apixaban. Furthermore, apixaban is significantly metabolized via sulfation, and roughly 25% of the dosage is eliminated in the urine as metabolites.
About 27% of the clearance of Apixaban comes from renal excretion; the remaining portion is removed predominantly as unaltered medication through the fecal route. Apixaban has a half-life of roughly 12 hours, which allows for a twice-daily dosage schedule. In patients with mild to moderate renal impairment, this balanced elimination process helps reduce the danger of buildup; nonetheless, in severe situations, dose changes may be required.
Apixaban’s pharmacokinetics also indicate a minimal possibility of drug-drug interactions, especially when contrasted with warfarin. However, as CYP3A4 and P-glycoprotein strong inducers or inhibitors can greatly alter Apixaban’s plasma concentrations, care must be used while taking them together.
Isotopically Labeled Apixaban and Research Applications
Apixaban molecules that have been isotopically tagged, like carbon-13 or deuterium-labeled variants, are essential for pharmaceutical research, especially when it comes to pharmacokinetics, drug metabolism, and safety evaluations. In preclinical and clinical investigations, these labeled analogs play a crucial role in tracking the absorption, distribution, metabolism, and excretion (ADME) of apixaban.
The investigation of Apixaban’s metabolic pathways is one of the main uses for isotopically labeled medication. Through the substitution of certain atoms in the Apixaban molecule with their isotopic counterparts, researchers may more precisely monitor the drug’s metabolic changes. This aids in comprehending the synthesis of both active and inactive metabolites and in locating putative metabolic pathways that might have unfavorable outcomes.
Furthermore, these labeled substances are necessary for bioanalytical procedures, especially when creating assays with high sensitivity and specificity. In liquid chromatography-mass spectrometry (LC-MS) experiments, isotopically labeled Apixaban acts as an internal standard, guaranteeing precise measurement of the medication and its metabolites in biological samples. This is essential for figuring out pharmacokinetic parameters and evaluating how the medication behaves in various populations, such as individuals with various levels of hepatic or renal impairment.
Apart from their application in pharmacokinetics and metabolic investigations, isotopically labeled Apixaban molecules hold significance in investigations concerning drug-drug interactions. Predicting Apixaban’s interactions with other drugs is a critical task that these studies aid in, as it helps to ensure patient safety and optimize treatment plans.
Current Research and Future Perspectives
With an emphasis on enhancing its safety profile and broadening its therapeutic applications, current research on Apixaban aims to explore its potential beyond the current prescriptions. The use of Apixaban in individuals with cancer-associated thrombosis is one intriguing area of research. Thromboembolic events are a much higher risk for cancer patients. Although apixaban is now utilized in these circumstances, studies are being conducted to better understand the safety and efficacy of this high-risk population’s medication. The purpose of these trials is to ascertain whether apixaban, rather than low molecular weight heparins, which are now the recommended course of treatment, can be an anticoagulant of choice for cancer patients.
The possible application of apixaban in combination therapy is an additional topic of investigation. To increase Apixaban’s efficacy in reducing thrombotic events, especially in patients with acute coronary syndromes or undergoing percutaneous coronary procedures, researchers are looking at possible combinations with other anticoagulants or antiplatelet medicines. Combinations like this might lower the risk of unfavorable cardiovascular events while keeping bleeding risks under control.
Another key area of current research is the development of reversal drugs for Apixaban. While the risk of bleeding events associated with Apixaban is lower than that of warfarin, having an efficient reversal agent on hand is crucial in case of emergency. While the development of andexanet alfa, a particular antidote for Factor Xa inhibitors such as Apixaban, is a noteworthy development in this field, further study is required to maximize its application and investigate alternative reversal tactics.
Researchers are also looking into the pharmacogenomics of Apixaban as personalized medicine develops to learn more about how genetic differences may affect the drug’s pharmacokinetics and pharmacodynamics. The findings of this study may result in more individualized dosage schedules, which would increase Apixaban’s effectiveness and safety for specific patients.
Conclusion
Due to its many advantages over conventional choices like warfarin, apixaban has become an indispensable part of anticoagulant therapy. It is a recommended option for the treatment and prevention of thromboembolic events due to its good safety profile, predictable pharmacokinetics, and focused mechanism of action. The continuous dedication to improving the efficacy and safety of apixaban is evident in the ongoing research activities focused on broadening its applications, refining combination therapies, and creating potent reversal medicines.
With the potential for new therapeutic applications and more individualized methods to anticoagulant management, apixaban’s future in clinical practice is bright. Apixaban is probably going to be at the vanguard of anticoagulant therapy, helping to enhance patient outcomes in a range of circumstances, as long as the medical community keeps investigating these possibilities.
In conclusion, apixaban has significantly improved the treatment of thromboembolic illnesses, and as long as research on the drug’s potential uses is conducted, it should have a bright future.




