Apoptosis Pathways and Targets
Abstract
Apoptosis is a basic biological phenomenon of cells and plays a necessary role in the removal of unwanted or abnormal cells by multicellular organisms. It plays an important role in the evolution of organisms, the stability of the internal environment, and the development of multiple systems. Apoptosis is not only a special type of cell death but also has important biological significance and complex molecular biological mechanisms.
What is apoptosis
The cells in the human body are destined to die, some of the death is physiological, and some of the death is pathological, the study of the cell death process has become a hot spot in biological and medical research. There are at least two known ways of cell death: apoptosis and necrosis. Cell necrosis is a recognized mode of cell death, while apoptosis is a gradually recognized mode of cell death.
Apoptosis is a basic biological phenomenon of cells and plays a necessary role in the removal of unwanted or abnormal cells by multicellular organisms. It plays an important role in the evolution of organisms, the stability of the internal environment, and the development of multiple systems. Apoptosis is not only a special type of cell death but also has important biological significance and complex molecular biological mechanisms.
Apoptosis is a process strictly controlled by multiple genes. These genes are very conserved between species, such as the Bcl-2 family, caspase family, oncogenes such as C-myc, tumor suppressor gene P53, etc. With the development of molecular biology techniques, the process of apoptosis of a variety of cells has been fairly understood, but the exact mechanism of the apoptosis process is still not completely clear up to now. The disorder of the apoptosis process may be directly or indirectly related to the occurrence of many diseases. For example, tumors, autoimmune diseases, and so on, can induce apoptosis of many factors, such as radiation, drugs, and so on.
What is the process of apoptosis?
The process of apoptosis can be roughly divided into the following stages:
Receiving the apoptotic signal → Interaction between apoptotic regulatory molecules → Activation of proteolytic enzymes (Caspase) → Entering the continuous reaction process
1. For the start stage: The initiation of apoptosis is the opening or closing of a series of intracellular control switches after the cells feel the corresponding signal stimulation. Different external factors initiate apoptosis in different ways and cause different signal transduction. Objectively speaking, the understanding of the signal transmission system in the process of apoptosis is still not comprehensive.
1.1 Membrane receptor pathway of cell apoptosis: various external factors are the initiators of cell apoptosis, which can transmit apoptotic signals through different signaling systems and cause cell apoptosis.
Fas is a transmembrane protein belonging to the tumor necrosis factor receptor superfamily, and its combination with FasL can initiate apoptosis signal transduction and cause apoptosis. Its activation involves a series of steps: the ligand-induced trimerization of the receptor is followed by the formation of an apoptosis-inducing complex on the cell membrane, which includes the Fas-associated protein FADD with a death domain. Fas, also known as CD95, is a receptor molecule composed of 325 amino acids. Once Fas binds to ligand FasL, it can initiate lethal signal transduction through Fas molecules and eventually cause a series of characteristic changes in cells, resulting in cell death. As a universally expressed receptor molecule, Fas can appear on the surface of a variety of cells, but FasL expression has its characteristics, usually only appearing in activated T cells and NK cells. Therefore, activated killer immune cells are often the most effective way to kill target cells through apoptosis. The intracellular segment of the Fas molecule has a special death domain (DD, death domain). After the combination of Fas and FasL, the death domains of the three Fas molecules gathered together into clusters and attracted another protein FADD with the same death domain in the cytoplasm. FADD is a connexin in death signal transcription, which consists of two parts: the C-terminal (DD domain) and the N-terminal (DED) part. The DD domain is responsible for binding with the DD domain on the intracellular segment of the Fas molecule, and the protein is connected with DED to another subsequent component. As a result, the DED of the N segment is then linked to the inactive cysteine protease 8 (caspase8), and multiple caspase8 molecules are polymerized. The caspase8 molecule then transforms from a single-stranded proenzyme into an active double-stranded protein, which in turn sets off a subsequent cascade of reactions, known as Caspases, which are activated as proenzymes and trigger the following cascade of reactions. The cells undergo apoptosis. Therefore, the TNF-induced apoptosis pathway is similar.
1.2 Biochemical pathways of cytochrome C release and Caspases activation
Mitochondria is the control center of cell life activities, which is not only the center of cell respiration chain and oxidative phosphorylation but also the center of cell apoptosis regulation. The results show that the release of cytochrome C from mitochondria is a key step in cell apoptosis. Cytochrome C release into the cytoplasm in the presence of dATP can bind to apoptosis-related factor 1 (Apaf-1) to form a polymer, and promote caspase-9 to bind to it to form apoptotic bodies, and caspase-9 is activated. Activated caspase-9 can activate other caspases, such as caspase-3, and thus induce cell apoptosis. In addition, mitochondria also release apoptosis-inducing factors, such as AIF, which are involved in the activation of caspase. It can be seen that the relevant components of apoptotic corpus exist in different parts of normal cells. Proapoptotic factors can induce the release of cytochrome C and the formation of apoptotic bodies. The regulation of cytochrome C release from mitochondria is a key issue in the study of the molecular mechanism of apoptosis. Most of the apoptotic stimulators activate the apoptotic pathway through mitochondria. It has been suggested that the receptor-mediated apoptosis pathway also involves the release of cytochrome C from mitochondria. For example, in cells responding to Fas, type 1 cell contains enough caspase8 to be activated by death receptors and lead to apoptosis. High expression of Bcl-2 in these cells did not inhibit Fas-induced apoptosis. In another class of cells (type2), such as hepatocytes, Fas receptor-mediated caspase-8 activation is not achieved at very high levels. Therefore, the apoptotic signal in these cells needs to be amplified by the mitochondrial pathway of apoptosis, and Bid, a Bcl-2 family protein containing only the BH3 domain, is the messenger that carries the apoptotic signal from caspase-8 to the mitochondria.
2. For the execution stage: Although the detailed mechanism of apoptosis is not fully understood, Caspase has been determined to play an essential role in the process of apoptosis, which is a cascade amplification reaction of irreversible limited hydrolyzed substrates of Caspase. Up to now, at least 14 caspase types have been discovered. Caspase has high molecular homology and similar structure, both of which are cysteine family proteases. According to function, Caspase can be divided into two categories: one is involved in cell processing, such as Pro-IL-1β and Pro-IL-1δ, forming active IL-1β and IL-1δ; The second group is involved in apoptosis, including caspase2,3,6,7,8,9.10.
Target about Apoptosis:
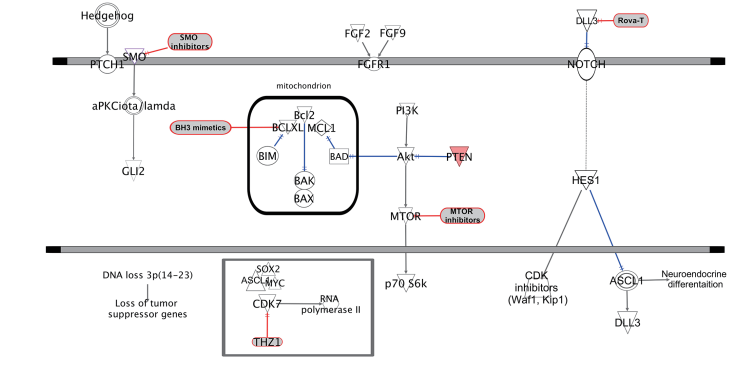
Bcl-2: The Bcl-2 protein family (B cell lymphoma/leukemia-2 protein) is a kind of protein with an inhibitory effect on cell apoptosis. Various signal transduction pathways of apoptosis share a common pathway or junction, and the junction of this pathway is regulated by Bcl-2. Bcl-2 family proteins are composed of pro-apoptotic factors (Bax, Bak, Bad, Bid, Puma, Bim, and Noxa) and anti-apoptotic factors (Bcl-2, Bcl-xL, Bcl-w, Mcl-1). Apoptosis is triggered when pro-apoptotic family members with BH3 domains, such as BAD, BID, and BIM, are activated during cellular stress through expression changes or post-translational modifications. Anti-apoptotic family members (e.g., Bcl-2, Bcl-XL, and Mcl-1) can bind and inhibit pro-apoptotic proteins to prevent apoptosis. These proteins are often active in cancer cells and are therefore attractive targets for the development of cancer therapeutics.
c-Myc: The proto-oncogene c-MYC is implicated in various physiological processes—cell growth, proliferation, loss of differentiation, and cell death (apoptosis). Oncogenic c-MYC implies constitutive or deregulated expression of c-MYC and is associated with many human cancers often with poor prognosis. Recently, c-MYC has been implicated in the loss and dysfunction of insulin-producing b cells in diabetes. Intriguingly, this raises the possibility that c-Myc may be a key contributor to disease, not only by deregulating cell proliferation, which is well established, but also by virtue of its opposing role in engendering apoptosis. However, given the fact that human diseases at diagnosis are generally advanced and pathologically complex, it is generally difficult to attribute a specific pathogenic role to c-MYC, or indeed any given single factor, or to assess the potential of therapies targeting individual such factors.
Caspase: Since the initial discovery of caspase activity over a decade ago, the confluence of genetics, biochemistry, and structural biology has led to rapid progress in understanding many facets of caspase function and regulation. It is now universally recognized that caspases are the key effectors of apoptosis, but many aspects of caspase biology present avenues for important discoveries. Some of the future challenges in the field include (i) unraveling new pathways of caspase activation, such as those triggered by oncogenes, disease protein aggregates, microbial infections, and lymphocyte negative selection; (ii) identifying new caspase substrates and how they contribute to the function of caspases; (iii) finding new mechanisms of caspase regulation (are there alternative mechanisms of procaspase activation and caspase cascade propagation?); (iv) developing selective small-molecule inhibitors, especially non-peptide-based inhibitors for pharmaceutical uses; and (v) defining new functions of caspases, such as in development, differentiation, antigen presentation, and others that may be separate from apoptosis. Based on the rapid progress to date, the coming years should witness many pharmaceutical discoveries about caspases.
IAP: Members of the mammalian inhibitor of apoptosis (IAP) family of proteins, including X chromosome-linked IAP (XIAP), cellular IAP 1 (cIAP1), cellular IAP 2 (cIAP2), and melanoma IAP (ML-IAP), are frequently overexpressed in cancer cells, where they confer protection against a variety of pro-apoptotic stimuli. The IAP proteins have also been demonstrated to function in the regulation of signal transduction pathways associated with malignancy. In particular, the cIAP proteins regulate TNFα-mediated NF-κB activation via their C-terminal RING ubiquitin E3-ligase domains, which have been shown to ubiquitinate receptor interacting protein (RIP)-1 and NF-κB inducing kinase, NIK. Efforts to target the IAP proteins have focused on the design of small molecules that mimic the binding of the endogenous IAP antagonist second mitochondria-derived activator of caspases/ direct IAP-binding protein with low pI (Smac/DIABLO) to a shallow groove on the surface of select IAP baculoviral IAP repeat (BIR) domains. The IAP BIR domains are approximately 80-amino acid zinc-binding domains that are necessary for the antiapoptotic function of the IAP proteins. The third BIR domain (BIR3) of XIAP is a specific inhibitor of caspase-9, while the BIR2 domain is necessary for potent inhibition of caspases-3 and -7. Antagonism of XIAP-mediated inhibition of these caspases is required for efficient caspase dependent cell death via both the extrinsic death receptor mediated and the intrinsic mitochondrial-mediated apoptosis pathways.
DAPK: In addition to DAPk, the DAPk protein family includes at least two other closely related kinases, DAPk2/DRP-1 (DRP-1 for DAPk-related protein. Not to be confused with dynamin-related protein also commonly abbreviated as DRP-1) and DAPk3/ZIPk/Dlk (ZIPk) sharing around 80% identity in their kinase domains. Interestingly, the extra-catalytic domain structures differ substantially from each other, suggesting that there should be some regulatory and functional divergence among these family members. In some models such as primary fibroblasts, overexpression of the DAPk family members induced caspase-dependent cell death with apoptotic characteristics like DNA fragmentation and caspase activation. However, in other cells, e.g., HeLa, MCF-7, or HEK 293 cells, overexpression of DAPk proteins led to a caspase-independent cell death.1Closer analysis of DAPk and DRP-1-induced morphological changes by electron microscopy revealed that HEK 293 or MCF-7 cells transfected with these kinases contain in their cytoplasm many autophagic vesicles and autolysosomes.1Furthermore, overexpression of DAPk, DRP-1 or ZIPk led to autophagic membrane association of the otherwise soluble autophagy marker GFP-LC3. From the molecular point of view, some of the well-documented pathways in the mode of action of DAPk, such as p53 activation or the cross-talk with ERK, highlight its function as a tumor suppressor gene. The detailed molecular mechanisms which link the different DAPk family members to autophagy still await further studies.
RIP kinase: In the past few years, much progress has been made towards understanding the functions of the different RIP family members. These RIP kinases share a similar kinase domain, yet they are also characterized by shared capacities to transmit intracellular responses to various environmental factors that we have collectively termed stress. They all have important roles in situations of cellular stress caused by different factors, such as pathogen infections, inflammation, cellular differentiation programs and DNA damage. Although these stimuli are different, they converge to initiate similar responses, for instance, activation of transcription factors such as NF-kB or AP-1, in addition to stimulating cell death. The tumour necrosis factor (TNF) superfamily of ligands and their receptors initiate different pathways that can, in general terms, lead to diverse responses such as cell survival or death. In 1995, the first member of the RIP family, RIP, was initially described as a novel kinase with a C-terminal death domain that can interact with the DD of one of these receptors, Fas, in a yeast two-hybrid screen, hence the designation ‘receptor-interacting protein’. RIP1 is constitutively expressed in many tissues, and also shows inducible expression upon T-cell activation and after stimulation with TNF. Subsequent studies have described the capacity of the DD of RIP to be recruited to several DD receptors. Importantly, RIP is recruited via the adaptor protein TNF-R-associated death domain (TRADD) to the TNF-receptor 1 (TNF-R1) upon ligand binding. RIP1 also interacts via the intermediate domain with TNF-receptor-associated factor (TRAF)-1, -2 and -3. Surprisingly, overexpression of RIP1 in 293 cells results in both the activation of an NF-kB–luciferase reporter plasmid and induction of cell death. Hence, a dual role for RIP1 was proposed: survival through NF-kB activation and cell-death induction.
TNF receptor: The tumor necrosis factor receptor (TNFR) superfamily represents a growing family, with over 20 members having been identified thus far in mammalian cells. These proteins share significant homologies in their extracellular ligand binding domains and intracellular e?ector (death) domains. These receptors appear to transmit their signals via protein-protein interactions, which convey either a death or survival signal. Isolation and characterization of death domain containing proteins (TRADD, FADD/MORT-1, RIP), TRAF domain containing proteins (TRAF1-6) as well as new members and adaptor proteins such as DAXX have provided new insights to our understanding of signaling mechanisms associated with this family of receptors. While the death signals seem to be associated with the activation of both the caspase and JUN kinase pathways, the survival signals are mediated via the activation of the NF-kB pathway.
Ferroptosis: Ferroptosis is a new type of iron-dependent programmed cell death, which is different from apoptosis, cell necrosis, and autophagy. The main mechanism of iron death is that under the action of bivalent iron or ester oxygenase, the high expression of unsaturated fatty acids on the cell membrane is catalyzed and lipid peroxidation occurs, thus inducing cell death. In addition, the expression of the antioxidant system (glutathione GSH and glutathione peroxidase 4-GPX4) was decreased. Iron death is a form of non-apoptotic cell death that depends on the accumulation of iron in cells and causes the increase of toxic lipid peroxide ROS. Tumor-associated fibroblasts (CAFs) can promote tumor progression and drug resistance by secreting a variety of bioactive substances, including exosomes. However, the role of CAFs in regulating lipid metabolism and iron death in tumor cells is still unknown, based on which researchers began to explore further.
Necroptosis: Necrotizing apoptosis is the pathway of programmed lysis cell death and is thought to play a role in killing pathogen-infected and/or damaged cells during certain degenerative or inflammatory diseases. Necrotizing apoptosis can be induced by a variety of innate immune signaling pathways, including those triggered by stimulation of RIG-I receptors, TLRS, and death receptors. These signaling pathways all lead to phosphorylation and activation of the necrotizing kinase RIPK3 (receptor-interacting serine-threonine kinase 3), which is also required for RIPK1 activity in the case of necrotizing apoptosis induced by the death receptor. RIPK3 activates the pseudokinase MLKL (mixed lineage kinase domain like) by phosphorylation, causing its conformational change and activation. The activated MLKL is localized to the plasma membrane and causes changes in membrane permeability. RIPK3 is activated by various extracellular and intracellular signals, either directly or through RIPK1. Ripk3-mediated phosphorylation induces translocation of the MLKL membrane and, as a result, ion influx leads to death. Survival signals protect cells from unnecessary necrosis by blocking RIPK1-induced signaling through upregulation of IAPs or activation of TAK1 kinase pathway. Caspase-8-mediated cleavage of pre-apoptotic RIPK1 and RIPK3 ensures the advantage of immunosilenced apoptosis over immunostimulated necrosis.
Pyroptosis: Pyroptosis is a form of programmed cell death associated with antimicrobial responses during inflammation. In contrast to apoptosis, pyroptosis requires the function of caspase-1, and has been studied in the context of salmonella-infected macrophages. The initiation of pyroptosis has been linked to the recognition of flagellin components of Salmonella and Shigella species (and similar pathogen-associated molecular patterns (PAMPs) in other microbial pathogens) by NOD-like receptors (NLRs). These receptors function similarly to plasma membrane Toll-like receptors (TLRs), but recognise intracytoplasmic antigens rather than extracellular antigens. Recently, it was shown that Caspase-1 is activated during pyroptosis by a large supramolecular complex termed the pyroptosome (also known as an inflammasome). Only one large pyroptosome is formed in each macrophage, within minutes after infection. Biochemical and Mass Spectroscopic analysis revealed that this pyroptosome is largely composed of dimers of the adaptor protein ASC. Unlike apoptosis, pyroptosis results in the release of pathogen associated molecular patterns (PAMPs) and cytokines that activate pro-inflammatory immune cell mediators.