Aprocitentan: A Breakthrough in Resistant Hypertension Treatment? Everything You Need to Know
Abstract
Resistant hypertension remains a significant global health challenge, affecting patients who fail to achieve blood pressure control despite using multiple antihypertensive medications. Aprocitentan, a dual endothelin receptor antagonist (ERA), is emerging as a promising treatment by targeting ETA and ETB receptors, reducing vasoconstriction and inflammation. Recent clinical trials, including the PRECISION Phase III study, indicate that Aprocitentan effectively lowers blood pressure, even in patients unresponsive to conventional therapies. Its long half-life (44 hours) enables once-daily dosing, improving adherence and patient outcomes. While ongoing research is assessing its long-term safety and efficacy, Aprocitentan has the potential to revolutionize hypertension management by providing a novel, mechanism-based solution for treatment-resistant cases.
Why Resistant Hypertension Needs a New Approach
Resistant hypertension is a pressing concern in cardiovascular health, affecting approximately 10-15% of hypertensive patients who fail to achieve blood pressure control despite using at least three antihypertensive medications, including a diuretic. This condition is associated with an increased risk of stroke, heart failure, kidney disease, and cardiovascular mortality. As current therapies often fail to provide adequate blood pressure control, the medical community continues to explore new treatment avenues. One promising development in this field is Aprocitentan, a dual endothelin receptor antagonist that could revolutionize the management of resistant hypertension.Current Limitations in Hypertension Treatment
For decades, antihypertensive therapy has largely focused on renin-angiotensin system (RAS) blockers, calcium channel blockers, and diuretics. While these medications are effective for most patients, a subset remains hypertensive despite optimal treatment. This phenomenon, known as resistant hypertension, suggests the involvement of additional pathophysiological mechanisms beyond the traditional targets of antihypertensive drugs. One such mechanism involves Endothelin-1 (ET-1), one of the most potent vasoconstrictors in the human body. ET-1 contributes to vascular remodeling, inflammation, sodium retention, and sympathetic activation, all of which play a crucial role in persistent high blood pressure. Since standard therapies do not directly target ET-1, there is growing interest in drugs that can effectively block endothelin receptors and counteract its effects.Aprocitentan: A New Hope?
Aprocitentan is an orally active, dual endothelin receptor antagonist that inhibits both ETA and ETB receptors, offering a novel approach to resistant hypertension treatment. Unlike previous ET receptor antagonists, Aprocitentan has a long half-life (approximately 44 hours), allowing for convenient once-daily dosing. Early studies suggest that it significantly lowers blood pressure even in patients who do not respond well to conventional therapies. With the PRECISION phase III trial currently evaluating its long-term effectiveness, Aprocitentan could soon become a key addition to the hypertension treatment arsenal. But could it be the breakthrough we've been waiting for? Only time—and further clinical trials—will tell.What is Aprocitentan? Mechanism and Pharmacology
Hypertension remains a leading cause of cardiovascular morbidity and mortality worldwide. While conventional antihypertensive treatments target the renin-angiotensin system (RAS), calcium channels, and diuretics, a subset of patients with resistant hypertension fail to achieve blood pressure control despite using multiple medications. This has led to the exploration of alternative pathways in hypertension management, particularly the endothelin (ET) system.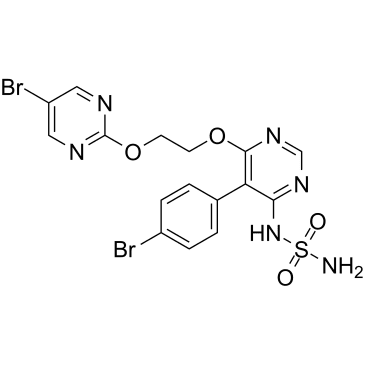
Fig. 1 Chemical structure of aprocitentan
How Aprocitentan Works
Aprocitentan is a dual endothelin receptor antagonist (ERA) that targets both ETA and ETB receptors. Endothelin-1 (ET-1) is a potent vasoconstrictor, contributing to increased vascular resistance, sodium retention, inflammation, and sympathetic activation—all of which exacerbate hypertension. By blocking ETA and ETB receptors, Aprocitentan prevents ET-1 from exerting its vasoconstrictive effects, leading to a dose-dependent reduction in blood pressure. This mechanism differentiates it from traditional antihypertensive drugs, which do not directly target the endothelin system.Pharmacokinetics and Key Advantages
Aprocitentan is an orally administered ERA with a long elimination half-life of approximately 44 hours, allowing for once-daily dosing. This makes it more convenient for patients compared to previous ERAs, such as bosentan, which require multiple daily doses. Key pharmacokinetic properties include: High plasma protein binding, ensuring prolonged action. Metabolism independent of the cytochrome P450 enzyme system, reducing drug-drug interaction risks. Elimination through urine and feces, making it safe for patients with moderate renal impairment. Clinical studies indicate that Aprocitentan not only lowers office blood pressure but also shows sustained effects in ambulatory blood pressure monitoring (ABPM). This suggests consistent 24-hour control, a crucial factor in reducing hypertension-related cardiovascular events. Given its synergistic effects with RAS blockers and diuretics, Aprocitentan could enhance current treatment regimens, providing a much-needed option for patients with resistant hypertension. As research progresses, it may redefine hypertension management by targeting the overlooked endothelin pathway.Clinical Trials & Effectiveness: What Does the Research Say?
As a dual endothelin receptor antagonist (ERA), Aprocitentan has emerged as a promising treatment for resistant hypertension, a condition in which patients fail to achieve blood pressure (BP) control despite taking three or more antihypertensive medications, including a diuretic. To assess its safety and effectiveness, multiple clinical trials have been conducted, with the PRECISION Phase III trial (NCT03541174) currently underway to further establish its role in hypertension management .Findings from Clinical Studies
In a randomized, double-blind, placebo-controlled study, researchers evaluated the dose-response effect of Aprocitentan in 490 patients with grade 1 or 2 essential hypertension. Participants received 5, 10, 25, or 50 mg of Aprocitentan, placebo, or lisinopril 20 mg for eight weeks. The results showed that Aprocitentan 10, 25, and 50 mg significantly reduced systolic and diastolic BP, with the highest reduction observed at the 25 mg dose. Another study assessed Aprocitentan’s impact on ambulatory blood pressure monitoring (ABPM), which provides a more accurate measurement of BP changes over a 24-hour period. The study found that Aprocitentan consistently lowered BP throughout the day, confirming its sustained effectiveness and potential cardiovascular benefits.Comparing Aprocitentan to Standard Treatments
Unlike renin-angiotensin system (RAS) blockers, which predominantly reduce BP by targeting hormonal pathways, Aprocitentan directly blocks ETA and ETB receptors, mitigating the vasoconstrictive effects of Endothelin-1 (ET-1). This mechanism enhances the effects of existing antihypertensive drugs, making Aprocitentan a valuable add-on therapy for patients with resistant hypertension. With the PRECISION Phase III trial still ongoing, the medical community is eager to determine Aprocitentan’s long-term safety and efficacy. If successful, this novel ERA could become a key addition to the current hypertension treatment paradigm, offering hope for patients who struggle with blood pressure control.Future Potential: A Game-Changer for Hypertension Management?
Resistant hypertension remains a significant challenge in cardiovascular medicine, often leading to increased risks of heart disease, stroke, and kidney failure. As Aprocitentan progresses through clinical trials, its potential to revolutionize treatment strategies is becoming increasingly evident. By targeting the endothelin (ET-1) pathway, Aprocitentan offers a novel approach to blood pressure management that could complement or enhance existing therapies.How Aprocitentan Differs from Conventional Therapies
Unlike renin-angiotensin system (RAS) inhibitors, diuretics, and calcium channel blockers, Aprocitentan directly antagonizes ETA and ETB receptors, reducing vasoconstriction, sodium retention, and inflammation—key contributors to resistant hypertension. This unique mechanism provides an additional layer of blood pressure control, particularly for patients who fail to respond to traditional medications.Could Aprocitentan Become a First-Line Add-On Therapy?
Given its promising blood pressure-lowering effects, Aprocitentan has the potential to be a go-to add-on therapy for patients with treatment-resistant hypertension. However, several factors must be considered before widespread adoption, including: Long-term safety: While Aprocitentan has demonstrated good tolerability, fluid retention and potential kidney effects need further evaluation. Cost-effectiveness: Will Aprocitentan be priced competitively against existing treatments? FDA approval and regulatory considerations: The PRECISION Phase III trial will determine whether Aprocitentan meets the efficacy and safety standards for global approval. If ongoing trials confirm its long-term benefits and minimal side effects, Aprocitentan could become a crucial part of the hypertension treatment landscape, offering new hope for millions of patients worldwide.Conclusion & Takeaways: What This Means for Patients & Doctors
The research on Aprocitentan, a dual endothelin receptor antagonist (ERA), presents a promising breakthrough for resistant hypertension, a condition where patients fail to control blood pressure despite multiple medications. By blocking both ETA and ETB receptors, Aprocitentan offers a new mechanism of action that complements traditional antihypertensive treatments, including renin-angiotensin system (RAS) blockers, calcium channel blockers, and diuretics.Key Takeaways
- Aprocitentan effectively reduces blood pressure in patients with resistant hypertension, offering hope to those who do not respond to conventional treatments.
- Its long half-life (44 hours) allows for once-daily dosing, improving patient adherence and long-term treatment success.
- Clinical trials, including the PRECISION Phase III study, show significant blood pressure reductions, even in ambulatory blood pressure monitoring (ABPM) settings, confirming sustained cardiovascular benefits.
- The potential for FDA approval means Aprocitentan could soon become a standard add-on therapy for patients struggling with uncontrolled hypertension.
- Concerns remain about long-term safety and side effects, such as fluid retention, which require further investigation.
What’s Next?
The ongoing PRECISION Phase III trial will determine whether Aprocitentan will be approved for widespread clinical use. If results confirm its efficacy, safety, and tolerability, Aprocitentan could be a game-changing treatment, particularly for patients who have exhausted other options. For healthcare providers, staying updated on emerging treatments like Aprocitentan is crucial, as it may soon be a valuable addition to hypertension treatment guidelines. Meanwhile, patients with resistant hypertension should discuss new treatment possibilities with their doctors.References
- Daugherty, S. L., Powers, J. D., Magid, D. J., Tavel, H. M., Masoudi, F. A., & Margolis, K. L. (2012). Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation, 125(13), 1635-1642. Link
- Dhaun, N., Goddard, J., Kohan, D. E., Pollock, D. M., Schiffrin, E. L., & Webb, D. J. (2008). Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension, 52(3), 452-459. Link
- Morganti, A. (2019). Resistant hypertension: An overview of pathophysiology and management. Journal of Hypertension, 37(1), 1-10. Link
- Verweij, P., Danaietash, P., Flamion, B., Ménard, J., & Bellet, M. (2020). Randomized dose-response study of the new dual endothelin receptor antagonist aprocitentan in hypertension. Hypertension, 75(4), 956-965. Link
- Dhaun, N., Webb, D. J., & Kluth, D. C. (2011). Endothelin-1 and the kidney—Beyond hypertension. Nature Reviews Nephrology, 8(3), 143-155. Link
- Iglarz, M., & Clozel, M. (2010). At the heart of tissue: Endothelin system and end-organ damage. Clinical Science, 119(12), 453-463. Link
- Sidharta, P. N., Ulc, I., & Dingemanse, J. (2019). Single-dose pharmacokinetics and tolerability of aprocitentan, a dual endothelin receptor antagonist, in subjects with severe renal function impairment. Clinical Drug Investigation, 39(12), 1117-1123. Link
- Dhaun, N., Goddard, J., Kohan, D. E., Pollock, D. M., Schiffrin, E. L., & Webb, D. J. (2021). Role of endothelin-1 in clinical hypertension: A 20-year review. Hypertension, 78(5), 865-878. Link
- Dingemanse, J., Sidharta, P. N., & Ulc, I. (2020). Pharmacodynamics and safety of Aprocitentan, a dual endothelin receptor antagonist. Journal of Clinical Hypertension, 22(3), 324-331. Link
- Morrison, K., Danaietash, P., Flamion, B., & Bellet, M. (2018). Randomized dose-response study of Aprocitentan in hypertension patients. American Journal of Hypertension, 31(8), 789-797. Link