Abstract
For the treatment of estrogen receptor-positive (ER+) breast cancer, which makes up a sizable portion of all occurrences of the illness, a novel oral selective estrogen receptor degrader (SERD), ARV-471, is being researched. ARV-471 circumvents the limitations of existing endocrine drugs, such as standard SERDs and SERMs, by selectively degrading estrogen receptors through the use of proteolysis-targeting chimera (PROTAC) technology. Positive outcomes from clinical trials have been noted, including significant reductions in estrogen receptor levels and tumor growth in patients not responding to current therapies. ARV-471 holds considerable promise as a transformational treatment for patients with ER+ breast cancer due to its distinct method of action and possible application in combination therapy and other hormone-driven malignancies.
Introduction to ARV-471: A Breakthrough in ER+ Breast Cancer Treatment
Around 70% of all occurrences of breast cancer are estrogen receptor-positive (ER+), making it one of the most prevalent cancers that strike women globally. Estrogen receptor activation drives ER+ breast cancer by encouraging tumor development. Although many patients’ outcomes have improved with contemporary medicines such as aromatase inhibitors and selective estrogen receptor modulators (SERMs), treatment failure frequently results from the development of resistance to these drugs over time.
Arvinas and Pfizer collaborated to develop ARV-471, a potentially effective novel treatment for ER+ breast cancer. ARV-471 is a proteolysis-targeting chimera (PROTAC) that does more than just block estrogen receptors; it also specifically destroys them. In contrast to traditional treatments, which might just inhibit the receptor’s function, ARV-471 targets the estrogen receptor for destruction by the cell’s own proteasome system. The process of degradation results in a notable decrease in the receptor’s presence within cancer cells, hence restricting their growth and proliferation.
ARV-471 may be able to get around resistance mechanisms linked to current treatments by directly destroying the estrogen receptor. Because of its novel strategy, ARV-471 is especially interesting for treating breast cancer patients who have become resistant to existing endocrine medications. With a notable decrease in ER levels and tolerable side effects, the early trial results are encouraging and suggest that ARV-471 may revolutionize the treatment of hormone-driven breast malignancies.
Mechanism of Action: How ARV-471 Works
When treating estrogen receptor-positive (ER+) breast cancer, ARV-471 is a state-of-the-art method. It’s a proteolysis-targeting chimera (PROTAC), a group of substances that specifically break down proteins inside of cells. Because the ER is a major factor in the development of ER+ breast cancer, ARV-471 is particularly engineered to destroy the ER. Although they block or decrease ER signaling, traditional medicines such as selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) do not completely eliminate the receptor from the cell, which can allow cancer to adapt and build resistance over time.
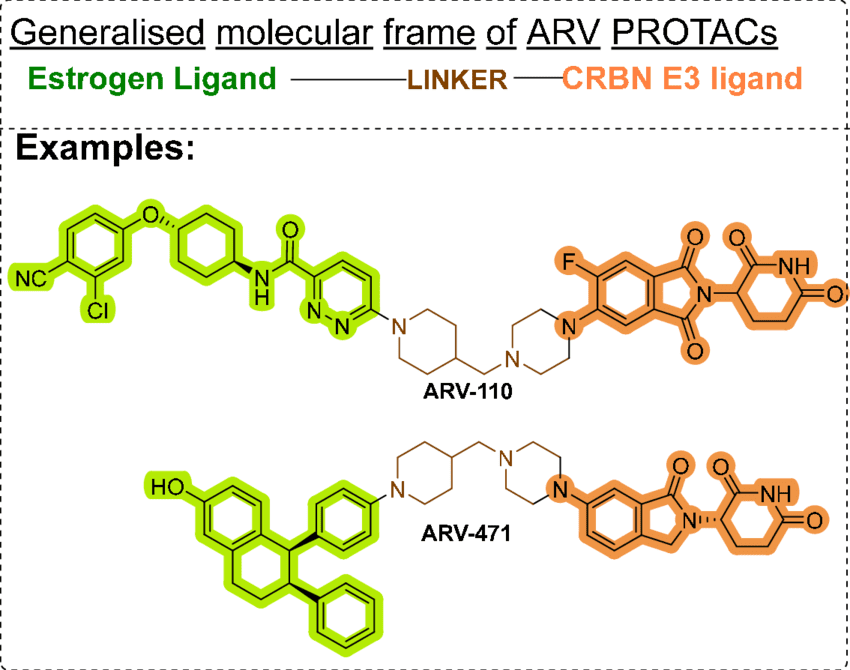
Fig.1 Molecular structure of ARV-110 and ARV-471
The mechanism of ARV-471 is distinct. It brings an E3 ubiquitin ligase complex and the estrogen receptor closer together by binding to both of them. This sets off the ubiquitination of the estrogen receptor, designating it for enzymatic breakdown by the proteasome, a mechanism found in cells. As a result, cancer cells have a marked decrease in the amount of estrogen receptors that are present, so blocking the signaling pathways that promote tumor growth.
Many of the shortcomings of current treatments can be addressed by ARV-471 since it degrades the estrogen receptor rather than inhibits it. By suppressing ER activity more thoroughly and persistently, this targeted degradation may lessen the chance of resistance developing. Early clinical trials using this unique technique have showed promise, with notable decreases in both tumor growth and ER levels noted, even in patients who had been resistant to prior treatments. Because ARV-471 has a stronger and longer-lasting therapeutic effect, it has the potential to completely change the way that ER+ breast cancer is treated.
Clinical Trials and Efficacy
With encouraging outcomes from its clinical development, ARV-471 is now positioned as a cutting-edge alternative for treating estrogen receptor-positive (ER+) breast cancer. Early-stage clinical trials have shown that ARV-471, even in patients who have become resistant to existing endocrine therapy, is effective in lowering estrogen receptor levels in patients with advanced or metastatic ER+ breast cancer. The treatment of breast cancer is hampered by patients’ resistance to medicines like aromatase inhibitors and selective estrogen receptor modulators (SERMs), which makes ARV-471’s potential all the more remarkable.
ARV-471 demonstrated excellent pharmacokinetics in the Phase 1/2 clinical trials, meaning that the medication was well absorbed and kept the body at effective levels. Notably, ARV-471-treated individuals showed notable decreases in estrogen receptor levels, which is essential for reducing tumor growth. Positive initial results regarding tumor response were also reported by the trials; other patients showed partial responses or stable illness. With the majority of adverse events being mild to moderate in severity, ARV-471’s safety profile has also been positive.
ARV-471’s clinical effectiveness raises the prospect of using it either alone or in conjunction with other targeted medicines. It is anticipated that ARV-471 will continue to demonstrate its efficacy in enhancing outcomes for patients with ER+ breast cancer as additional information from ongoing clinical trials becomes available, particularly for individuals who have few treatment options because of resistance to current medications.
Comparison with Existing SERDs
Compared to conventional selective estrogen receptor degraders (SERDs), ARV-471 has a number of advantages. The way that modern SERDs, like fulvestrant, function is by attaching to estrogen receptors and degrading them. Nevertheless, fulvestrant’s effectiveness is restricted due to its low absorption, necessitating intramuscular injections, and partial degradation of estrogen receptors, which results in less than ideal therapeutic results. Furthermore, fulvestrant resistance is a common occurrence in individuals, which limits their alternatives for treatment.
On the other hand, ARV-471, a PROTAC-based medication, offers a more effective and focused method of estrogen receptor degradation. In order to ensure a more thorough elimination of the receptor, ARV-471 not only attaches to the estrogen receptor but also activates the cell’s built-in protein-degradation machinery. ARV-471 is able to circumvent the drawbacks of fulvestrant, such as its limited bioavailability and partial receptor degradation, because to this approach. In addition, patients may find ARV-471 to be more convenient to take orally as opposed to injectable SERDs.
Because of this, ARV-471 may be able to treat ER+ breast cancer more successfully, particularly in those who have grown resistant to fulvestrant and other SERDs. Although further research is needed to fully understand the benefits of ARV-471, preliminary findings indicate that it significantly improves the therapeutic options available for hormone receptor-positive malignancies.
Future Directions and Potential Impact
ARV-471 appears to have a bright future in the treatment of breast cancer, especially as research into its full potential is still ongoing. ARV-471 is a selective estrogen receptor degrader (SERD) that can be taken orally. Its unique mechanism of action makes it a promising treatment option for patients with estrogen receptor-positive (ER+) breast cancer, especially those who have become resistant to existing endocrine medications. Current clinical trials are being expanded to encompass larger populations, and scientists are investigating its possible conjunction with other treatments, like immunotherapy and CDK4/6 inhibitors.
ARV-471 has potential applications outside breast cancer. Its applicability in other estrogen-driven cancers, such as ovarian and endometrial cancers, where estrogen receptors also significantly contribute to tumor growth, is being studied by researchers. Furthermore, the success of ARV-471 may open the door for the use of additional proteolysis-targeting chimeras (PROTACs) in cancer therapies that target additional important oncogenic proteins.
Impact-wise, ARV-471 has the potential to greatly enhance patient outcomes by providing a more practical and efficient therapeutic choice. Compared to existing injectable SERDs like fulvestrant, its oral administration is more patient-friendly, and its technique of degrading estrogen receptors may be able to overcome the problems associated with existing therapies’ resistance. ARV-471 may establish itself as a routine treatment for ER+ breast cancer if later-phase trials show promise. This would offer patients who have few treatment options because of resistance fresh hope.
Conclusion
ARV-471 represents a groundbreaking advancement in the treatment of ER+ breast cancer. Its ability to degrade estrogen receptors through a novel proteolysis-targeting chimera (PROTAC) mechanism sets it apart from traditional therapies like selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs). With early clinical trials showing promising results in terms of efficacy and safety, ARV-471 offers hope for patients who have developed resistance to current endocrine treatments.
As clinical research advances, ARV-471 has the potential to revolutionize breast cancer treatment by providing a more effective and focused therapeutic approach. Moreover, novel therapy options for oncology are made possible by its possible use in combination therapies and other tumors driven by estrogen. Numerous people with hormone-driven tumors may survive longer because to ARV-471, which offers an oral, safe, and effective substitute for current treatments.