ARV-766: A Promising PROTAC for Overcoming Resistance in Advanced Prostate Cancer
Abstract
A new oral PROTAC that degrades androgen receptors (AR) called ARV-766 was created to treat metastatic castration-resistant prostate cancer (mCRPC). AR L702H, T878, and H875, which are linked to resistance to traditional treatments, are examples of clinically significant AR mutations that ARV-766 targets and degrades using PROTAC technology. With notable decreases in prostate-specific antigen (PSA) levels and acceptable tolerability profiles shown in early clinical trials, ARV-766 appears to be a promising therapy option for advanced prostate cancer. ARV-766 is anticipated to be crucial in overcoming medication resistance and enhancing patient outcomes in mCRPC as research advances.
Introduction to ARV-766
Arvinas Inc. developed ARV-766, an experimental oral androgen receptor (AR) degrading PROTAC (Proteolysis Targeting Chimera). It is intended to treat aggressive metastatic castration-resistant prostate cancer (mCRPC), which frequently develops resistance to conventional androgen receptor-targeted treatments. ARV-766 functions by focusing on and destroying the AR protein itself, which is a major factor in the advancement of prostate cancer, in contrast to conventional treatments that block AR activity.
Targeting wild-type AR as well as clinically significant AR mutations, like the AR L702H mutation, which are frequently linked to resistance to existing treatments, is one of ARV-766’s special advantages. As a result, patients who have grown resistant to first-generation AR inhibitors may find ARV-766 to be a more viable option.
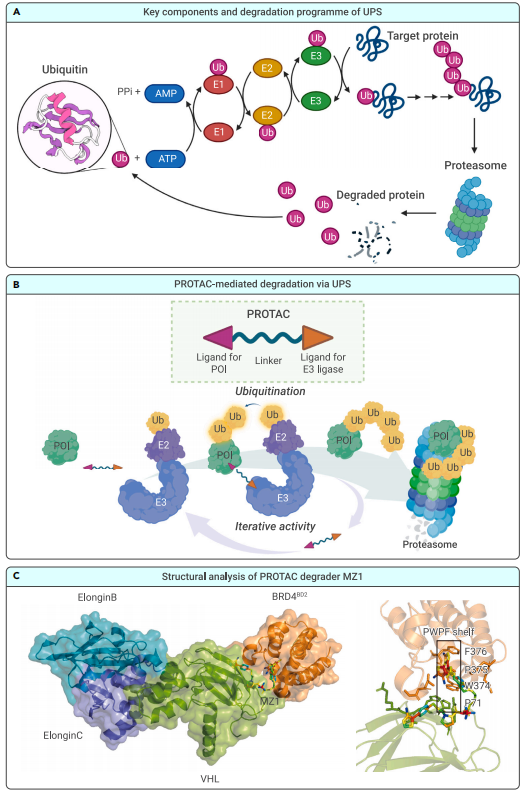
The core of the mechanism of ARV-766 is PROTAC technology. By attracting the androgen receptor to the cell’s natural protein degradation machinery, the proteasome, it helps to selectively degrade the androgen receptor. Compared to conventional AR antagonists, this method is more effective since it not only suppresses the receptor but also eliminates it completely from the body.
ARV-766 clinical trials have produced positive outcomes. A considerable proportion of patients in early-phase trials showed a decrease in prostate-specific antigen (PSA) levels, which is a gauge of prostate cancer activity. The majority of treatment-related adverse events have been mild to moderate, indicating that the chemical has also been well tolerated.
For individuals with advanced prostate cancer, ARV-766 offers hope for better results by presenting a novel therapeutic approach to overcome resistance in the illness.
Mechanism of Action of ARV-766
The new mechanism by which ARV-766 works is known as PROTAC (Proteolysis Targeting Chimera) technology, which is intended to break down androgen receptors (AR), which are essential for the advancement of prostate cancer. By attracting the androgen receptor to the proteasome, the cell’s natural degradation mechanism, ARV-766 completely destroys the receptor in contrast to conventional AR antagonists, which just inhibit the androgen receptor’s function. Because of this focused degradation, the cancer cells are less likely to become resistant to traditional AR inhibitors, which is a prevalent issue.
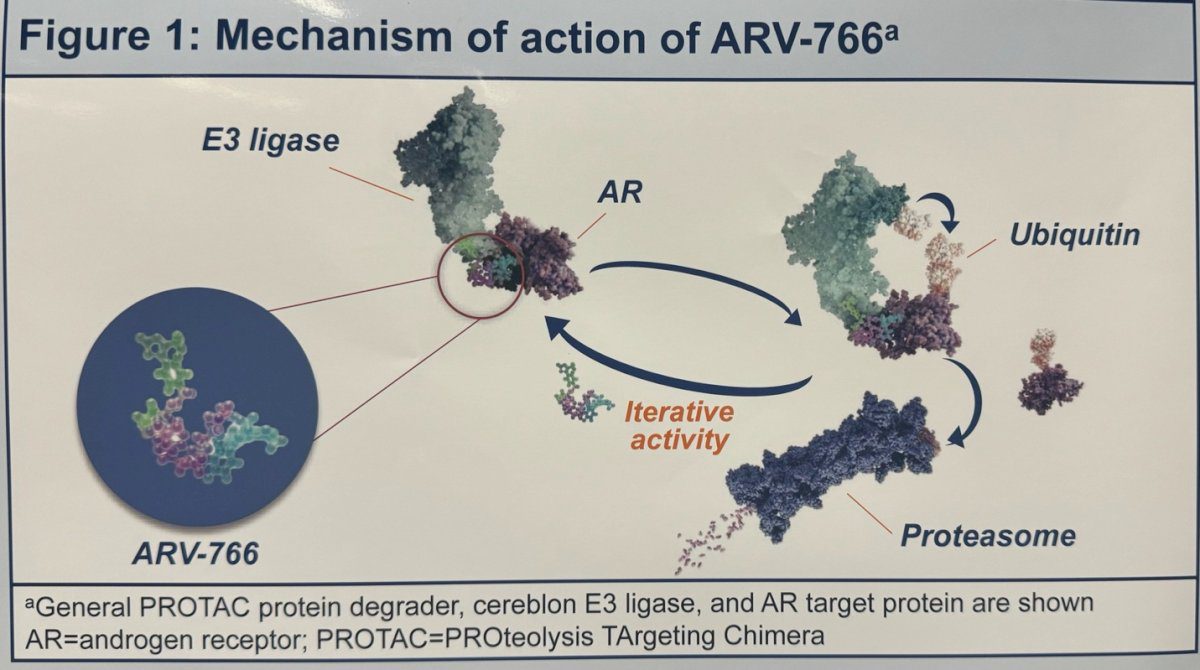
Fig.1 Mechanism of Action of ARV-766
Because ARV-766 may degrade clinically relevant mutations including AR L702H, T878A, and H875Y in addition to wild-type androgen receptors, it provides a more reliable treatment option for patients with advanced prostate cancer, even those who have not responded to first-line therapy. Resistance to androgen-deprivation therapy (ADT), a common treatment for prostate cancer, is frequently linked to mutations in the androgen receptor. ARV-766 provides a possibly better way to tackle this problem by selectively destroying these mutant receptors.
The mechanism is related to PROTACs’ bifunctional structure. ARV-766 has two ends: one that binds to the androgen receptor and the other that enlists the help of an E3 ubiquitin ligase to tag the AR protein for proteasome destruction. Rather than only blocking AR signaling, this strategy guarantees an ongoing elimination of the AR, providing a more thorough means of stifling AR-induced tumor growth. ARV-766 targets a wider variety of cancer cells by destroying both mutant and wild-type AR, which lowers the chance that the disease will advance.When AR mutations have rendered conventional medicines ineffective for treating prostate cancer, this degradation process offers a potentially effective new therapeutic approach.
Clinical Significance of ARV-766
A noteworthy development in the management of metastatic castration-resistant prostate cancer (mCRPC), a subtype of the disease that develops resistance to conventional androgen deprivation therapy (ADT), is ARV-766. ARV-766, a next-generation PROTAC (Proteolysis Targeting Chimera), addresses a significant issue in the treatment of prostate cancer: resistance caused by mutations in the androgen receptor (AR). It does this by completely degrading the receptor.
Targeting wild-type and clinically significant AR variants, including AR L702H, T878, and H875 mutations, is one of ARV-766’s most significant features. Patients with mCRPC have previously had fewer therapy options due to the development of resistance to first-line AR-targeted treatments, which is often linked to these mutations. ARV-766 provides a more comprehensive and potent therapy strategy by inhibiting both wild-type and mutant androgen receptors.
Clinical trials have yielded positive results: a considerable proportion of patients have demonstrated a decrease in prostate-specific antigen (PSA) levels, a crucial biomarker for tracking the advancement of prostate cancer. ARV-766 showed significant anti-tumor activity in early-phase trials, as evidenced by a PSA reduction of 50% or greater in a significant proportion of patients. Moreover, the medication has been well-tolerated; the majority of treatment-related adverse events (TRAEs), like nausea and exhaustion, have been mild to moderate in nature.
ARV-766 is anticipated to be a useful treatment for patients with mCRPC, especially those who have run out of alternative therapeutic options, due to its mechanism of action and clinical efficacy. It is a good option for enhancing prognoses in cases of advanced prostate cancer because of its capacity to overcome resistance to conventional therapy.
Clinical Trials and Efficacy Data
Clinical trials involving ARV-766 show that it may be a useful treatment for metastatic castration-resistant prostate cancer (mCRPC). Significant efficacy has been demonstrated by the Phase 1 and Phase 2 trials, especially in individuals with AR mutations who are resistant to conventional androgen receptor inhibitors. AR L702H, T878, and H875, among other variants, are frequently associated with treatment failure in current therapeutic interventions.
ARV-766 has demonstrated encouraging outcomes in early trials, with a significant proportion of patients reporting a 50% or greater drop in prostate-specific antigen (PSA). Given that PSA levels and the advancement of prostate cancer are positively connected, this biomarker is significant. To be more precise, this decrease was seen in about 42% of the study participants, demonstrating the compound’s efficacy in reducing tumor activity. Furthermore, partial reductions in tumor size were seen in individuals whose tumors included AR mutations; these responses included both confirmed and unconfirmed responses, underscoring ARV-766’s possible anti-tumor actions.
As ARV-766 progresses through clinical trials, interest has been drawn to its potential to prevent medication resistance while preserving safety. Based on the results of the clinical studies, ARV-766 is generally well tolerated; most treatment-related adverse events (TRAEs), such as fatigue, nausea, and diarrhea, are Grade 1 or 2. It is anticipated that more patients with advanced prostate cancer would benefit from ARV-766 when its safety and efficacy profile is further established through ongoing trials, especially the extension of Phase 2.
Safety Profile and Tolerability
Throughout its clinical trials, ARV-766 has demonstrated a good safety profile, with the majority of treatment-related side events having a mild to moderate severity. Of the 34 patients in the Phase 1 dose-escalation experiment, only two needed dose reductions because of side effects associated to the medication. No patient had dose-limiting toxicities.
The trials have shown that fatigue, nausea, and diarrhea are common adverse effects; nevertheless, there have been no reports of treatment-related adverse events (TRAEs) of Grade 4 or higher, indicating that ARV-766 is a tolerable medication. Moreover, most of these episodes were mild and temporary, enabling most patients to resume their treatments without difficulty. The positive safety profile of ARV-766 indicates that it may be a good choice for long-term treatment in patients with prostate cancer and encourages its further development.
Future Prospects of ARV-766
Future clinical trials are anticipated to further establish ARV-766’s status as a unique therapeutic in the treatment of metastatic castration-resistant prostate cancer (mCRPC). Future studies on ARV-766 should focus on how it might be used in combo treatments. Preliminary findings indicate that ARV-766 may work well in combination with medications like the androgen biosynthesis inhibitor abiraterone to improve treatment outcomes for people with advanced prostate cancer.
The start of an international Phase 3 trial aimed at verifying ARV-766’s effectiveness in a broader patient base is another area of exploration. If this experiment is successful, regulatory approvals and eventual commercialization may follow. The substance is already demonstrating great promise in treating drug-resistant AR mutations, which continue to be a serious therapy challenge for prostate cancer.
ARV-766 may also prove to be a useful tool in the individualized therapy of cancer. It is a candidate for precision medicine strategies because of its capacity to target particular AR mutations, enabling the customization of therapy according to the unique characteristics of each patient. This is consistent with the expanding oncology trend of customized therapy, in which medications are created to specifically target the distinct biological traits of each patient’s tumor.
In addition to improving prognoses for mCRPC patients, the successful development of ARV-766 may open the door for its application in prostate cancer patients who may benefit from early intervention to avoid resistance to conventional therapies.
Conclusion
An important development in the management of metastatic castration-resistant prostate cancer (mCRPC) is ARV-766. It differs from conventional AR inhibitors due to its unique mode of action, which uses PROTAC technology to destroy the androgen receptor (AR). ARV-766 successfully targets two of the most important issues in the treatment of prostate cancer: resistance to current therapies. It does this by targeting both wild-type AR and clinically relevant AR mutations, such as AR L702H, T878, and H875.
Clinical trials have shown that ARV-766 has the ability to meaningfully improve response rates in patients with advanced, drug-resistant prostate cancer and to dramatically lower PSA levels. Due to its ability to target AR mutations and its acceptable safety profile, the chemical is a very intriguing option for use in upcoming therapies for prostate cancer.
With the ongoing development of ARV-766 and the impending Phase 3 studies, the medication may soon be used as routine treatment for mCRPC. Its use in tailored treatment plans and combination medicines points to its extensive applicability in a variety of prostate cancer situations. ARV-766 has the potential to change the oncology landscape by enhancing treatment outcomes and providing hope to patients who have run out of other alternatives.
References
- Zhang Y, Ming A, Wang J, Chen W, Fang Z. PROTACs targeting androgen receptor signaling: Potential therapeutic agents for castration-resistant prostate cancer. Pharmacol Res. 2024 Jul;205:107234. doi: 10.1016/j.phrs.2024.107234. Epub 2024 May 28. PMID: 38815882.
- Snyder, L., Lee, S. H., Neklesa, T. K., Chen, X., Dong, H., Ferraro, C., … & Taylor, I. (2023). Abstract ND03: Discovery of ARV-766, an androgen receptor degrading PROTAC® for the treatment of men with metastatic castration resistant prostate cancer. Cancer Research, 83(7_Supplement), ND03-ND03.