Aurora Kinase B: Unlocking New Horizons in Cancer Therapy
Abstract
Aurora Kinase B (AURKB) has emerged as a significant target in cancer therapy due to its critical role in mitosis and its frequent overexpression in multiple tumor types. AURKB is involved in chromosome alignment, segregation, and cytokinesis, making it essential for cancer cell proliferation and survival. Research into AURKB inhibitors, including barasertib and hesperadin, has demonstrated promising results in preclinical and clinical studies, although challenges remain due to off-target effects and the development of drug resistance. Combining AURKB inhibitors with agents such as PARP inhibitors or chemotherapeutics may enhance their efficacy and combat resistance mechanisms. Innovations like nanoparticle-based drug delivery, exemplified by AZD2811, have shown potential in improving drug targeting and reducing side effects. Future perspectives suggest that personalized medicine approaches, including genomic profiling, could optimize AURKB-targeted therapies for specific patient populations. This review underscores the importance of AURKB as a therapeutic target and the advancements needed to fully integrate AURKB inhibitors into cancer treatment.
Introduction to Aurora Kinase B and Its Role in Cancer
Aurora Kinase B (AURKB) is a serine/threonine protein kinase that plays a central role in the regulation of mitosis and is part of the chromosomal passenger complex (CPC), along with other key proteins like INCENP, survivin, and borealin. AURKB is essential for several mitotic processes, including chromosomal alignment, segregation, and cytokinesis, and it helps ensure accurate cell division. In normal cells, AURKB carefully regulates the phosphorylation of histone H3, which is crucial for chromosomal condensation. However, overexpression or deregulation of AURKB is often associated with tumorigenesis.
Research has highlighted the overexpression of AURKB in multiple cancers, including lung, colorectal, breast, and liver cancers, linking its elevated levels to increased cellular proliferation, genomic instability, and resistance to traditional therapies . This connection arises because AURKB overactivity can drive aneuploidy—abnormal chromosome numbers—contributing to the uncontrolled growth characteristic of cancer cells. Furthermore, elevated AURKB expression has been associated with poor prognosis in patients with non-small cell lung carcinoma (NSCLC) and colorectal cancer, underscoring its importance as a potential therapeutic target.
Given AURKB’s critical role in cell cycle regulation and its association with multiple cancers, targeting this kinase has become a promising therapeutic strategy. Inhibitors developed to suppress AURKB activity, such as hesperadin and barasertib, show potential in clinical settings by selectively interrupting the cell division of cancer cells, leading to cell death. Moreover, studies have suggested that combining AURKB inhibitors with other cancer therapies could help overcome drug resistance, thereby improving treatment outcomes. As research continues to elucidate the pathways and interactions involving AURKB, it stands out as a key target in developing advanced cancer therapies.
Mechanisms of AURKB in Cancer Progression
Aurora Kinase B (AURKB) is an essential protein for cell division, specifically for mitosis, where it ensures accurate chromosomal alignment, segregation, and cytokinesis. As a member of the chromosomal passenger complex (CPC), AURKB works with other proteins to promote proper chromosomal structure and attachment during cell division. In normal cells, AURKB activates and regulates histone H3 phosphorylation, which is critical for chromosome condensation and the maintenance of genomic stability. However, in many cancer types, AURKB expression is significantly upregulated, which disrupts the cell cycle, leading to genomic instability, aneuploidy, and uncontrolled proliferation.
This deregulation makes AURKB a driver of tumor progression, as it allows cancer cells to evade normal cell cycle checkpoints, leading to tumorigenesis. Studies have shown that elevated levels of AURKB are associated with poor prognosis in various cancers, including breast, lung, and colorectal cancers. For instance, in non-small cell lung cancer (NSCLC), AURKB overexpression is linked to increased resistance to chemotherapy, contributing to treatment challenges in these patients. The mechanism behind this involves AURKB’s role in maintaining spindle assembly checkpoint fidelity, which allows cancer cells to survive despite chromosomal missegregation events.
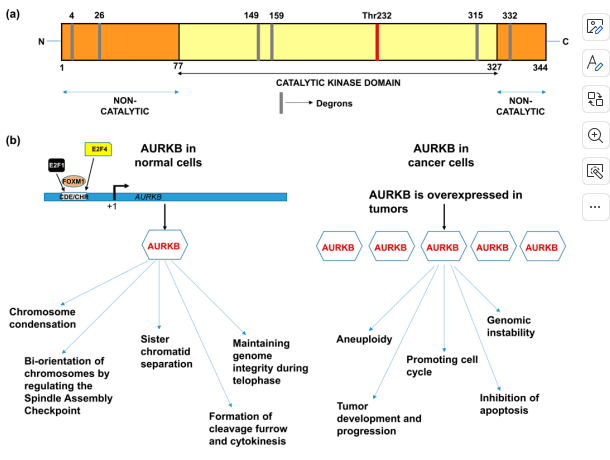
Figure 1. (a) Domain structure of AURKB showing catalytic and non-catalytic domains.(b) Functions of AURKB in normal cells and cancer cells.
Moreover, AURKB influences several oncogenic pathways by interacting with proteins like c-Myc and p53, both of which play crucial roles in cancer progression. AURKB phosphorylates these proteins, influencing their stability and function, further promoting a pro-cancerous environment by preventing apoptosis and enhancing tumor cell survival. As a result, AURKB’s role in both cell cycle progression and interaction with key oncogenes underscores its potential as a target for cancer therapies, with inhibitors developed to disrupt its activity and restore cell cycle control.
AURKB Inhibitors: Development and Clinical Trials
The overexpression and critical role of Aurora Kinase B (AURKB) in cancer progression have spurred the development of specific inhibitors targeting this kinase. AURKB inhibitors are designed to disrupt its mitotic functions, leading to cell cycle arrest and apoptosis in cancer cells. Among the first AURKB inhibitors developed were hesperadin and ZM447439, both of which showed efficacy in selectively blocking AURKB activity, leading to abnormal mitosis and polyploidy in cancer cells. These early inhibitors laid the groundwork for further research into AURKB-targeting drugs, revealing the potential of AURKB inhibition as a therapeutic strategy.
One of the most promising AURKB inhibitors to date is barasertib (AZD1152), a potent ATP-competitive inhibitor with high selectivity for AURKB. Barasertib induces cell cycle arrest in the G2/M phase, leading to cancer cell apoptosis, and has shown efficacy in preclinical studies on various cancer types, including acute myeloid leukemia (AML) and non-small cell lung cancer. Clinical trials of barasertib have demonstrated manageable toxicity levels, with neutropenia being the most common dose-limiting side effect.
Other AURKB inhibitors, such as GSK1070916 and AMG900, are currently being explored in clinical trials. GSK1070916 has shown potent inhibition of AURKB and AURKC, with a strong anti-tumor effect in various preclinical cancer models. AMG900, an orally administered pan-Aurora kinase inhibitor, has demonstrated efficacy in both solid tumors and hematologic cancers, with manageable toxicity when used with supportive therapies like granulocyte-colony stimulating factor (G-CSF).
Despite promising preclinical and clinical findings, challenges remain, particularly concerning the off-target effects and resistance mechanisms that may limit long-term efficacy. Nonetheless, AURKB inhibitors represent a promising avenue in cancer therapeutics, with ongoing studies aimed at improving their selectivity and developing combination therapies to maximize their anti-cancer potential.
Overcoming Drug Resistance with AURKB Inhibition
One of the most significant challenges in cancer treatment is the emergence of drug resistance, which often results in poor patient outcomes. Aurora Kinase B (AURKB) overexpression has been linked to drug resistance across several cancer types, including lung, breast, and colorectal cancers, where it allows cancer cells to bypass cell cycle checkpoints, evade apoptosis, and continue proliferating despite treatment. This association has made AURKB a target of interest for overcoming resistance, as inhibiting AURKB can restore the sensitivity of cancer cells to chemotherapy and other therapies.
AURKB inhibition has shown promise in overcoming resistance to specific targeted therapies, such as epidermal growth factor receptor (EGFR) inhibitors in non-small cell lung cancer (NSCLC). Studies have demonstrated that AURKB inhibitors, such as barasertib, can enhance the effects of EGFR inhibitors, allowing resistant NSCLC cells to respond to treatment once again. Additionally, in breast cancer, AURKB has been identified as a mediator of resistance to endocrine therapies, such as tamoxifen and fulvestrant. Targeting AURKB in these settings helps reduce the survival of resistant breast cancer cells, making it a promising approach for patients with hormone-resistant breast cancer.
The mechanism behind this effect is thought to involve AURKB’s role in regulating the spindle assembly checkpoint, a critical component of mitosis. By inhibiting AURKB, cells are more likely to undergo mitotic failure, leading to apoptosis, especially in cancer cells that have developed resistance mechanisms. Furthermore, recent studies suggest that combining AURKB inhibitors with other agents, like PARP inhibitors, can exert synergistic effects, particularly in tumors with DNA repair deficiencies.
Despite promising data, resistance to AURKB inhibitors themselves has been observed, often due to mutations within the kinase domain. Addressing these issues will require further research to develop more potent inhibitors or combination therapies that reduce resistance likelihood.
Future Perspectives on AURKB as a Cancer Therapeutic Target
Aurora Kinase B (AURKB) has emerged as a promising therapeutic target in cancer treatment due to its critical role in mitotic regulation and its overexpression in many cancers. Although several AURKB inhibitors, such as barasertib and hesperadin, have shown potential in preclinical and clinical studies, they face limitations related to selectivity, off-target effects, and resistance mechanisms. Addressing these challenges will require the development of more specific AURKB inhibitors and innovative therapeutic approaches, including combination therapies and nanoparticle-based drug delivery systems, to maximize efficacy and minimize toxicity.
One promising avenue for enhancing AURKB inhibitors’ effectiveness is through combination therapies that target multiple cancer pathways simultaneously. For instance, pairing AURKB inhibitors with PARP inhibitors has shown synergistic effects, especially in cancers with DNA repair deficiencies, by inducing synthetic lethality in tumor cells. Similarly, combining AURKB inhibitors with chemotherapeutic agents like gemcitabine and oxaliplatin may help overcome resistance in cancers such as pancreatic and colon cancer. Such strategies could enhance AURKB inhibitors’ therapeutic impact and potentially address drug resistance.
Nanotechnology also holds promise in optimizing AURKB inhibitor delivery. The development of nanoparticle formulations, like AZD2811, has improved drug stability, targeted delivery, and bioavailability, potentially reducing adverse effects seen with traditional administration methods. This approach allows for more precise targeting of cancer cells and a reduction in side effects, making AURKB inhibition safer and more effective for patients.
Lastly, personalized medicine approaches, including genomic profiling of tumors, can help identify patients most likely to benefit from AURKB inhibition. By targeting patient populations with specific genetic mutations or markers associated with AURKB overexpression, clinicians can tailor therapies for greater efficacy. As our understanding of AURKB and its role in cancer biology deepens, continued research and innovation will be essential in realizing its full therapeutic potential in oncology.
References
- Borah, N. A., & Reddy, M. M. (2021). Aurora Kinase B inhibition: A potential therapeutic strategy for cancer. Molecules, 26, 1981.
- Bertran-Alamillo, J., et al. (2019). AURKB as a target in non-small cell lung cancer with acquired resistance to anti-EGFR therapy. Nature Communications, 10(1), 1–14.
- Floc’H, N., et al. (2017). Optimizing therapeutic effect of Aurora B inhibition in acute myeloid leukemia with AZD2811 nanoparticles. Molecular Cancer Therapeutics, 16(6), 1031–1040.
- Floc’H, N., et al. (2019). Modeling dose and schedule effects of AZD2811 nanoparticles targeting Aurora B kinase for treatment of diffuse large B-cell lymphoma. Molecular Cancer Therapeutics, 18(5), 909–919.
- Gully, C. P., et al. (2012). Aurora B kinase phosphorylates and instigates degradation of p53. Proceedings of the National Academy of Sciences, 109(8), 1513–1522.
- Wilkinson, R. W., et al. (2007). AZD1152, a selective inhibitor of Aurora B kinase, inhibits human tumor xenograft growth by inducing apoptosis. Clinical Cancer Research, 13(12), 3682–3688.
- Adams, N. D., et al. (2010). Discovery of GSK1070916, a potent and selective inhibitor of Aurora B/C kinase. Journal of Medicinal Chemistry, 53(10), 3973–4001.
- Kantarjian, H. M., et al. (2017). A phase 1 study of AMG 900, an orally administered pan-aurora kinase inhibitor, in adult patients with acute myeloid leukemia. American Journal of Hematology, 92(7), 660–667.




