Axitinib for Renal Cell Carcinoma: Mechanism, Uses, Side Effects & Latest Research
Abstract
Axitinib (Inlyta) is a potent and selective tyrosine kinase inhibitor (TKI) approved for the treatment of advanced renal cell carcinoma (RCC). By targeting VEGF receptors (VEGFR-1, -2, and -3), Axitinib effectively inhibits angiogenesis — the formation of new blood vessels that tumors rely on for growth. Unlike traditional chemotherapy, Axitinib offers a more targeted approach, resulting in fewer systemic side effects. It is commonly used as a second-line therapy and has also shown strong efficacy when combined with immunotherapies such as pembrolizumab and avelumab, now forming part of first-line treatment options in metastatic RCC. Clinical trials such as KEYNOTE-426 and JAVELIN Renal 101 have demonstrated improved survival outcomes with these combinations. Ongoing research is exploring its broader application in other cancers. This blog provides an in-depth overview of Axitinib’s mechanism of action, medical indications, side effects, and its evolving role in combination therapies.
Introduction: What is Axitinib and Why is it Important in Cancer Treatment?
Axitinib, sold under the brand name Inlyta, is a powerful targeted therapy used primarily in the treatment of advanced renal cell carcinoma (RCC), the most common type of kidney cancer in adults. As a member of the tyrosine kinase inhibitor (TKI) class of drugs, Axitinib works differently from traditional chemotherapy — it’s designed to block specific proteins that help tumors grow and spread. Approved by the U.S. Food and Drug Administration (FDA) in 2012, it has since become a valuable second-line treatment option for patients whose cancer has progressed after initial therapy.
What sets Axitinib apart is its focus on angiogenesis inhibition — the process of preventing the formation of new blood vessels that supply nutrients to tumors. It selectively targets vascular endothelial growth factor receptors (VEGFR-1, VEGFR-2, and VEGFR-3), disrupting the tumor’s blood supply and slowing its growth. This precision-based mechanism means it often causes fewer systemic side effects compared to traditional chemotherapy, making it an appealing option for oncologists and patients alike.
In recent years, Axitinib has also shown promise when used in combination with immune checkpoint inhibitors such as pembrolizumab, expanding its role in first-line therapy. With an increasing body of clinical evidence supporting its efficacy, Axitinib represents a major advancement in the personalized treatment of kidney cancer — and is a subject of ongoing research for broader oncological applications.
As a VEGFR inhibitor, Axitinib offers a more targeted approach than traditional chemotherapy, which can affect both healthy and cancerous cells. This more selective action results in fewer side effects compared to chemotherapy. Over the years, clinical trials have demonstrated Axitinib’s efficacy in prolonging progression-free survival in patients with RCC. Moreover, research is ongoing to explore its potential in combination with immunotherapies for broader applications in treating other cancers.
Axitinib represents a critical advancement in the treatment of kidney cancer, offering hope for patients facing advanced stages of the disease.
How Axitinib Works (Mechanism of Action)
Axitinib works by inhibiting the activity of specific enzymes known as tyrosine kinases, which are involved in critical cell signaling pathways that regulate angiogenesis — the growth of new blood vessels. Tumors rely heavily on angiogenesis to receive nutrients and oxygen to support their rapid growth. By blocking this process, Axitinib essentially “starves” the tumor.
More specifically, Axitinib targets vascular endothelial growth factor receptors (VEGFR-1, VEGFR-2, and VEGFR-3). These receptors, when activated by VEGF proteins, signal endothelial cells to form new blood vessels. Axitinib binds to these receptors and inhibits their activity, thereby suppressing blood vessel formation in and around the tumor.
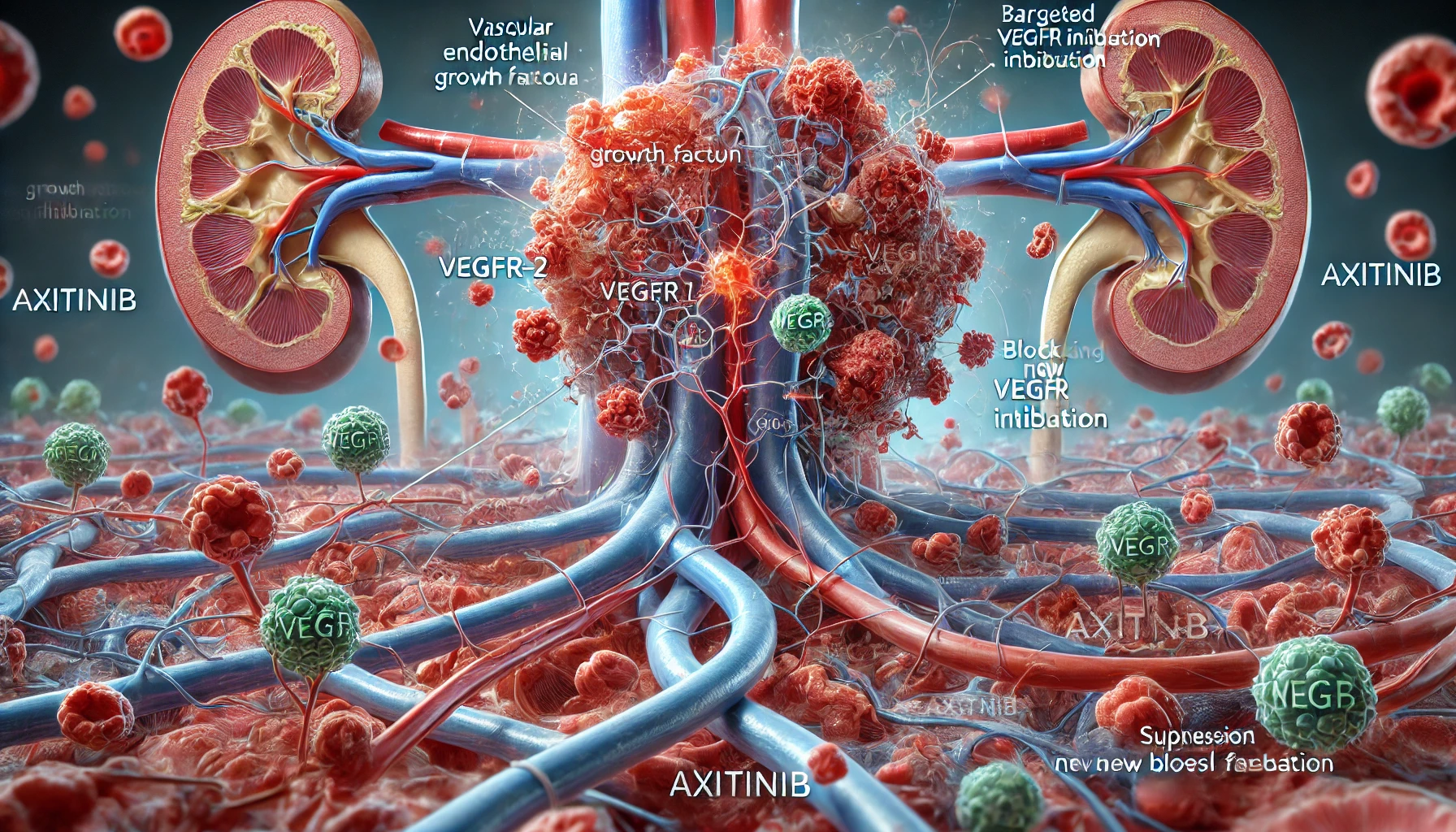
Unlike conventional chemotherapy that kills fast-dividing cells indiscriminately (often leading to widespread side effects), Axitinib’s precision targeting allows for fewer off-target effects. This makes it a more tolerable option for many patients, especially when used as part of long-term cancer management strategies.
This mechanism also makes Axitinib particularly suitable for combination therapies. When paired with immune checkpoint inhibitors like pembrolizumab, the inhibition of angiogenesis can enhance immune cell infiltration into tumors, making immunotherapy more effective.
In summary, Axitinib’s targeted disruption of VEGFR-mediated angiogenesis represents a crucial step in modern oncology, especially in the treatment of advanced kidney cancer.
Medical Uses and Indications
Axitinib is primarily approved for the treatment of advanced or metastatic renal cell carcinoma (RCC). RCC accounts for approximately 90% of kidney cancer cases, and many patients are diagnosed at an advanced stage where surgery alone is insufficient. Axitinib is typically prescribed when the disease progresses following the use of one prior systemic therapy, making it a common second-line treatment.
In clinical trials, Axitinib has shown to significantly prolong progression-free survival compared to other agents like sorafenib. It has since gained attention not only as a monotherapy but also in combination with immunotherapies. The KEYNOTE-426 trial, for instance, evaluated Axitinib with pembrolizumab (an anti-PD-1 antibody), demonstrating improved outcomes as a first-line treatment option for advanced RCC.
Besides RCC, Axitinib is being investigated in clinical trials for its potential use in other solid tumors, such as hepatocellular carcinoma, thyroid cancer, and certain subtypes of lung and breast cancer. However, these applications are still under research and have not yet received formal approval.
The flexibility of Axitinib, particularly its oral administration, makes it suitable for outpatient treatment settings, improving patient convenience. Its use is also expanding in personalized medicine, where genetic and molecular tumor profiling helps guide therapy decisions.
Side Effects and Warnings
Like all cancer therapies, Axitinib comes with potential side effects. While many patients tolerate it better than traditional chemotherapy due to its targeted nature, side effects still occur — some of which can be serious if not managed appropriately.
Common Side Effects:
Fatigue
Diarrhea
Hypertension (high blood pressure)
Nausea
Weight loss
Hand-foot syndrome (redness, pain, or blistering on hands and feet)
Hoarseness
Serious Risks and Warnings:
Hypertensive crisis: Axitinib can significantly elevate blood pressure, which must be monitored regularly.
Thromboembolic events: Increased risk of blood clots and strokes in some patients.
Bleeding: Axitinib may raise the risk of hemorrhagic events, particularly in patients with underlying vascular abnormalities.
Hepatotoxicity: Liver enzymes should be checked regularly, as liver damage is a potential concern.
Proteinuria: Loss of protein in the urine, which may indicate kidney dysfunction.
Axitinib is not recommended for patients with uncontrolled hypertension, recent significant bleeding, or severe liver impairment. Women of childbearing age should use effective contraception, as the drug can harm a developing fetus.
Proper dose adjustment and close monitoring are essential to managing side effects. Physicians often reduce the dosage or interrupt treatment if side effects become severe.
While these side effects may sound intimidating, many are manageable with prompt care. Open communication between patients and providers is critical for safe and effective treatment.
Axitinib in Combination Therapy
In recent years, combination therapy has revolutionized cancer treatment — and Axitinib is a cornerstone in this evolving strategy. When paired with immune checkpoint inhibitors, Axitinib enhances anti-tumor immune activity and improves patient outcomes.
Mechanistic Synergy:
Axitinib works by inhibiting angiogenesis, which not only starves tumors but also improves immune cell access to the tumor microenvironment. This makes tumors more responsive to immunotherapy agents such as:
Pembrolizumab (anti-PD-1)
Avelumab (anti-PD-L1)
These drugs “release the brakes” on the immune system, enabling T-cells to recognize and destroy cancer cells. Axitinib, by altering tumor blood vessels and decreasing immunosuppressive signals, enhances this effect.
Clinical Trial Success:
KEYNOTE-426 trial (Axitinib + Pembrolizumab): Showed superior overall survival and progression-free survival compared to sunitinib alone.
JAVELIN Renal 101 trial (Axitinib + Avelumab): Demonstrated significant improvement in outcomes in advanced RCC patients.
These results led to FDA approvals of Axitinib-based combination regimens as first-line treatment for metastatic RCC. The combinations are now standard in clinical guidelines.
Ongoing Research:
Axitinib is currently under investigation in combination with other therapies, including chemotherapy and novel immunomodulators, in lung, breast, and liver cancers.
The future of Axitinib lies in its ability to integrate into personalized multi-modal therapy, making it a key player in the next generation of oncology care.
Conclusion: Axitinib’s Evolving Role in Cancer Therapy
Axitinib has emerged as a vital component in the fight against advanced renal cell carcinoma and represents a broader shift toward targeted, personalized cancer therapies. Its ability to precisely inhibit vascular endothelial growth factor receptors (VEGFRs) makes it especially effective at halting tumor growth by disrupting the tumor’s blood supply — a hallmark of aggressive cancers.
What distinguishes Axitinib is not only its efficacy as a standalone second-line therapy, but also its groundbreaking use in combination with immunotherapies. Trials like KEYNOTE-426 and JAVELIN Renal 101 have propelled Axitinib into first-line treatment protocols, showcasing its ability to significantly improve both survival outcomes and quality of life for patients with metastatic kidney cancer. These advances have led to updates in international treatment guidelines, further solidifying its role in standard oncology care.
Additionally, ongoing research is expanding Axitinib’s potential into other tumor types and novel drug combinations. As our understanding of tumor biology and immune interactions deepens, Axitinib may play an even greater role in multi-modal treatment strategies for solid tumors beyond kidney cancer.
For patients and healthcare providers navigating the complexities of cancer treatment, Axitinib offers a blend of effectiveness, precision, and flexibility, making it a cornerstone in the era of precision oncology. As clinical research continues, its future looks promising — not only as a reliable treatment for RCC, but also as a key player in the next generation of cancer care innovation.
References
Ferrarotto R, Sousa LG, Feng L, Mott F, Blumenschein G, Altan M, Bell D, Bonini F, Li K, Marques-Piubelli ML, Dal Lago EA, Johnson JJ, Mitani Y, Godoy M, Lee A, Kupferman M, Hanna E, Glisson BS, Elamin Y, El-Naggar A. Phase II Clinical Trial of Axitinib and Avelumab in Patients With Recurrent/Metastatic Adenoid Cystic Carcinoma. J Clin Oncol. 2023 May 20;41(15):2843-2851. doi: 10.1200/JCO.22.02221. Epub 2023 Mar 10. PMID: 36898078; PMCID: PMC10414730.
https://pubmed.ncbi.nlm.nih.gov/36898078/
Lian B, Li Z, Wu N, Li M, Chen X, Zheng H, Gao M, Wang D, Sheng X, Tian H, Si L, Chi Z, Wang X, Lai Y, Sun T, Zhang Q, Kong Y, Long GV, Guo J, Cui C. Phase II clinical trial of neoadjuvant anti-PD-1 (toripalimab) combined with axitinib in resectable mucosal melanoma. Ann Oncol. 2024 Feb;35(2):211-220. doi: 10.1016/j.annonc.2023.10.793. Epub 2023 Nov 11. PMID: 37956739.
https://pubmed.ncbi.nlm.nih.gov/37956739/
Jiang H, Liao J, Wang L, Jin C, Mo J, Xiang S. The multikinase inhibitor axitinib in the treatment of advanced hepatocellular carcinoma: the current clinical applications and the molecular mechanisms. Front Immunol. 2023 May 31;14:1163967. doi: 10.3389/fimmu.2023.1163967. PMID: 37325670; PMCID: PMC10264605.
https://pubmed.ncbi.nlm.nih.gov/37325670/
Sharma A, Bahl A, Frazer R, Godhania E, Halfpenny N, Hartl K, Heldt D, McGrane J, Şahbaz Gülser S, Venugopal B, Ritchie A, Crichton K. Axitinib after Treatment Failure with Sunitinib or Cytokines in Advanced Renal Cell Carcinoma-Systematic Literature Review of Clinical and Real-World Evidence. Cancers (Basel). 2024 Jul 30;16(15):2706. doi: 10.3390/cancers16152706. PMID: 39123435; PMCID: PMC11312084.
https://pubmed.ncbi.nlm.nih.gov/39123435/
Jiao T, Wang Y, Lin X, Song W, Wang L, Rahman TMS, Xu L, Nie L, Zhang Q, Li J. Axitinib targets cardiac fibrosis in pressure overload-induced heart failure through VEGFA-KDR pathway. Front Med (Lausanne). 2023 Nov 10;10:1256156. doi: 10.3389/fmed.2023.1256156. PMID: 38020087; PMCID: PMC10667428.