Boosting ε-Poly-L-Lysine Production: How ATP Regeneration Is Transforming Microbial Fermentation
Abstract
ε-Poly-L-lysine (ε-PL) is a widely used natural antimicrobial agent, but its commercial production is often limited by low fermentation yields due to high intracellular ATP demand. A recent study introduced a novel metabolic engineering approach by integrating a polyphosphate kinase (PPK)-mediated ATP regeneration system into Streptomyces albulus. By coexpressing polyP:AMP phosphotransferase (PAP) and PPK2Bcg, the engineered strain significantly enhanced intracellular ATP levels and achieved a record ε-PL production of 59.25 g/L in fed-batch fermentation. This strategy offers a scalable, energy-efficient solution for overcoming biosynthetic bottlenecks and sets a new standard for high-yield microbial biomanufacturing.
Why ε-Poly-L-Lysine (ε-PL) Needs a Boost
ε-Poly-L-lysine (ε-PL) has emerged as one of the most promising natural preservatives in the food, pharmaceutical, and cosmetic industries. Produced by certain strains of Streptomyces, ε-PL is a biodegradable, non-toxic, and heat-stable homopoly(amino acid) that exhibits broad-spectrum antimicrobial activity. Its safety profile has led to its classification as Generally Recognized As Safe (GRAS) by the U.S. FDA, and its commercial use has grown rapidly in Japan, China, the United States, and South Korea.
Despite its advantages, industrial-scale production of ε-PL faces a major challenge: low fermentation yields. Naturally occurring strains typically produce less than 0.2 g/L of ε-PL, far below the thresholds required for cost-effective biomanufacturing. This low yield is primarily attributed to the high ATP demand of ε-PL biosynthesis, where each lysine monomer addition consumes one molecule of ATP. As fermentation progresses and intracellular ATP becomes depleted, ε-PL synthesis stalls, even when other nutrients remain available.
Over the years, several approaches have been implemented to improve ε-PL yields. These include traditional mutagenesis and resistance screening, as well as modern metabolic engineering techniques such as overexpression of hemoglobin genes (to enhance oxygen uptake) and synthetic pathways that improve L-lysine precursor availability. While helpful, these strategies often overlook the root issue—energy limitation at the cellular level.
In this context, recent innovations aim to directly address ATP scarcity by engineering ATP regeneration systems within the microbial host. Such systems promise a sustainable way to boost intracellular energy availability without the excessive energy input required by high-stirring or oxygenation techniques. By solving the ATP bottleneck, researchers can unlock higher ε-PL yields and transform microbial fermentation into a more viable industrial process.
The ATP Problem in ε-PL Biosynthesis
At the heart of ε-poly-L-lysine (ε-PL) production lies a fundamental energy challenge. The biosynthesis of this valuable biopolymer is uniquely ATP-intensive. Each lysine unit added to the growing ε-PL chain consumes one molecule of ATP, making the process one of the most energy-demanding among microbial secondary metabolites. As a result, intracellular ATP depletion becomes a critical bottleneck that limits ε-PL productivity, even in genetically optimized Streptomyces strains.
Under normal fermentation conditions, oxygen is essential for ATP generation through oxidative phosphorylation. However, Streptomyces albulus and similar filamentous bacteria create highly viscous broths due to their hyphal structure, reducing oxygen transfer efficiency. This leads to compromised energy production and stunted ε-PL biosynthesis. Even with robust oxygenation strategies, such as high-speed stirring or sparging, the physical limits of oxygen solubility and mechanical stress on cells impose diminishing returns.
Researchers have explored several solutions to bypass oxygen-related limitations. These include the overexpression of vgb (Vitreoscilla hemoglobin) to enhance cellular oxygen uptake and the use of oxygen carriers like n-dodecane to maintain higher dissolved oxygen levels. While such methods can improve ATP supply to a degree, they often involve high energy inputs and increased operational complexity in bioreactors.
Crucially, these approaches all address ATP demand indirectly—by focusing on oxygen delivery rather than ATP availability itself. Yet studies show that simply increasing oxygen levels doesn’t always correlate with a proportional increase in intracellular ATP or ε-PL yield. This underscores the need for a more targeted strategy that ensures continuous ATP regeneration within the cell.
By directly enhancing the cell’s ability to regenerate ATP, it becomes possible to bypass the limitations of oxygen transfer altogether. This approach not only has the potential to improve ε-PL production but could also pave the way for more efficient biosynthesis of other energy-demanding products.
A Novel Approach: Engineering ATP Regeneration with Polyphosphate Kinase
To address the bottleneck of intracellular ATP depletion during ε-poly-L-lysine (ε-PL) biosynthesis, researchers have shifted focus from traditional oxygen-based strategies to an innovative metabolic engineering solution: introducing an ATP regeneration system using polyphosphate kinase (PPK).
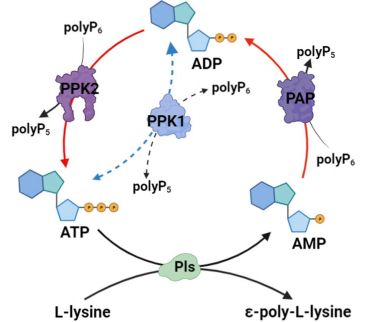
Fig. 1 PPK-mediated ATP regeneration system coupled with ɛ-PL biosynthesis
This approach is grounded in the concept of continuously replenishing ATP within the microbial cell through a cascade reaction. In the study by Yang et al., scientists engineered a high-yield ε-PL-producing strain of Streptomyces albulus by integrating two key enzymes into its genome: polyphosphate:AMP phosphotransferase (PAP) from Acinetobacter johnsonii, which converts AMP to ADP, and PPK2Bcg from Corynebacterium glutamicum, which catalyzes the final step from ADP to ATP using inexpensive polyphosphate (polyP) as a phosphate donor.
This engineered cascade—AMP → ADP → ATP—creates a self-sustaining cycle of energy regeneration. The resulting strain, named PL05, exhibited remarkable performance. In shake-flask fermentation, it produced 2.34 g/L ε-PL, representing a 21.24% increase over the wild-type, and intracellular ATP levels rose by 71.56%. These improvements are even more striking in fed-batch fermentation, where with optimized polyP6 supplementation, strain PL05 reached 59.25 g/L ε-PL, the highest yield ever recorded in a genetically engineered S. albulus strain.
Unlike earlier attempts that relied on oxygenation or precursor pathway enhancement, this method directly stabilizes cellular energy levels, making it more robust and adaptable. Notably, the use of polyP6 as the phosphate donor is both cost-effective and scalable, further supporting its industrial viability.
This breakthrough underscores a new paradigm in microbial fermentation—regenerating energy instead of just supplying it. As a result, the PPK-mediated ATP regeneration strategy opens new doors not only for enhanced ε-PL production but also for other biosynthetic pathways limited by energy constraints.
Why This Matters for Industrial Fermentation
The integration of a polyphosphate kinase-mediated ATP regeneration system into Streptomyces albulus is more than a clever laboratory innovation—it represents a major leap forward for industrial fermentation processes. ε-Poly-L-lysine (ε-PL) is widely used in the food, pharmaceutical, and cosmetic industries due to its natural antimicrobial properties, but until now, its production at scale has been restricted by energy constraints and metabolic inefficiencies.
With traditional fermentation strategies, improving ε-PL yields required either intense oxygenation (to drive oxidative phosphorylation) or random mutagenesis approaches, both of which come with limitations. High-speed stirring, for instance, increases energy costs and shear stress on microbial cells. Meanwhile, mutation-based strain development is time-consuming and unpredictable. These methods also fail to directly address the core issue—insufficient intracellular ATP during extended fermentation.
By contrast, the ATP regeneration strategy offers a game-changing solution. It allows cells to recycle AMP and ADP into ATP continuously, bypassing the need for excessive oxygen input and enabling more stable fermentation conditions. This not only improves ε-PL yield but also enhances fermentation robustness, cell viability, and resource efficiency.
The successful performance of the engineered strain PL05—reaching a record 59.25 g/L ε-PL in fed-batch fermentation—demonstrates the scalability and industrial relevance of this method. The use of polyphosphate (polyP6) as a phosphate donor adds another layer of practicality: polyP is affordable, stable, and easy to handle at scale.
Moreover, this strategy has cross-application potential. Many microbial products—such as antibiotics, amino acids, nucleotides, and certain vitamins—also suffer from ATP limitations during biosynthesis. By introducing tailored ATP regeneration systems, these processes too could see substantial productivity improvements.
In sum, this approach marks a paradigm shift in microbial bioproduction, one that prioritizes intracellular energy dynamics as a driver for enhanced yield and economic viability.
Conclusion: A Roadmap for the Future of Biomanufacturing
The development of an ATP regeneration system in Streptomyces albulus represents a transformative advancement in microbial biomanufacturing. Rather than relying solely on traditional methods—such as mutagenesis, pH shock, or oxygen optimization—this strategy targets the energetic foundation of biosynthesis itself: intracellular ATP availability. By integrating a simple yet powerful cascade using polyphosphate kinase (PPK2Bcg) and polyP:AMP phosphotransferase (PAP), researchers achieved unprecedented efficiency in ε-poly-L-lysine (ε-PL) production.
This work sets the stage for a new generation of strain engineering, one that doesn’t just enhance metabolic pathways but supports them with the energy required to perform at maximum capacity. The fact that ε-PL production in the engineered strain PL05 reached 59.25 g/L, the highest yield reported to date, underlines the potential of ATP regeneration as a core tool in synthetic biology and industrial microbiology.
Looking forward, this concept is highly adaptable. Any fermentation process that relies heavily on ATP—such as the production of glutamine, SAM (S-adenosylmethionine), nucleotides, or polyketides—could benefit from a similar approach. Coupling metabolic engineering with energy circuit optimization will allow bioengineers to build more resilient, high-yielding microbial cell factories.
Furthermore, the use of cost-effective and scalable inputs like polyphosphate (polyP6) ensures that this method remains industrially viable. Its compatibility with fed-batch fermentation systems further supports its utility in large-scale operations.
As the biomanufacturing industry seeks more sustainable and economically feasible production methods, targeting ATP regeneration will be an essential frontier. It allows for smarter use of resources, reduces dependency on energy-intensive oxygenation systems, and provides a modular solution that can be adapted across product lines.
This study doesn’t just solve a problem—it redefines how we think about energy management in biological systems and opens the door to a more efficient, bio-based future.
References
Hamano, Y., Arai, T., Ashiuchi, M., & Kino, K. (2013). NRPSs and amide ligases producing homopoly(amino acid)s and homooligo(amino acid)s. Natural Product Reports, 30(8), 1087–1097.
https://doi.org/10.1039/c3np20009k
Xu, Z., Feng, X., & Xu, H. (2016). Recent advances in the biotechnological production of microbial poly(ɛ-L-lysine) and understanding of its biosynthetic mechanism. Applied Microbiology and Biotechnology, 100(15), 6619–6630.
https://doi.org/10.1007/s00253-016-7544-4
Wang, L., Zhang, C. Y., Rao, Z. M., & Chen, X. S. (2021). Epsilon-poly-L-lysine: Recent advances in biomanufacturing and applications. Frontiers in Bioengineering and Biotechnology, 9, 748976.
https://doi.org/10.3389/fbioe.2021.748976
Yamanaka, K., Kito, N., Imokawa, Y., & Hamano, Y. (2010). Mechanism of epsilon-poly-L-lysine production and accumulation revealed by identification and analysis of an epsilon-poly-L-lysine-degrading enzyme. Applied and Environmental Microbiology, 76(17), 5669–5675.
https://doi.org/10.1128/AEM.00476-10
Xu, Z., Cao, C., Sun, Z., & Feng, X. (2015). Construction of a genetic system for Streptomyces albulus PD-1 and improving ε-poly-L-lysine production through expression of Vitreoscilla hemoglobin. Journal of Microbiology and Biotechnology, 25(11), 1819–1826.
https://doi.org/10.4014/jmb.1505.05041
Man, Z., Guo, J., Zhang, Y., & Cai, Z. (2020). Regulation of intracellular ATP supply and its application in industrial biotechnology. Critical Reviews in Biotechnology, 40(8), 1151–1162.
https://doi.org/10.1080/07388551.2020.1795613
Meng, L., Li, M., Yang, S. H., Kim, T. J., & Suh, J. W. (2011). Intracellular ATP levels affect secondary metabolite production in Streptomyces spp. Bioscience, Biotechnology, and Biochemistry, 75(8), 1576–1581.
https://doi.org/10.1271/bbb.110254
Yang, H., Zhu, D., Kai, L., Wang, L., Zhang, H., Zhang, J., & Chen, X. (2023). Engineering Streptomyces albulus to enhance ε-poly-L-lysine production by introducing a polyphosphate kinase-mediated ATP regeneration system. Microbial Cell Factories, 22(1), 51.
https://doi.org/10.1186/s12934-023-02057-7
Lv, Q., Hu, M., Tian, L., Liu, F., Wang, Q., Xu, M., & Rao, Z. (2021). Enhancing L-glutamine production in Corynebacterium glutamicum by rational metabolic engineering combined with a two-stage pH control strategy. Bioresource Technology, 341, 125799.
https://doi.org/10.1016/j.biortech.2021.125799
Chen, H., & Zhang, Y. (2020). Enzymatic regeneration and conservation of ATP: Challenges and opportunities. Critical Reviews in Biotechnology, 41(1), 16–33.
https://doi.org/10.1080/07388551.2020.1793862
Tavanti, M., Hosford, J., Lloyd, R. C., & Brown, M. J. (2021). ATP regeneration by a single polyphosphate kinase powers multigram-scale aldehyde synthesis in vitro. Green Chemistry, 23(2), 828–837.
https://doi.org/10.1039/D0GC03870K
Wang, L., Zhang, J., Mao, Z., & Chen, X. (2019). Efficiently activated ε-poly-L-lysine production by multiple antibiotic-resistance mutations and acidic pH shock optimization in Streptomyces albulus. MicrobiologyOpen, 8(1), e00728.
https://doi.org/10.1002/mbo3.728
Chen, Y. W., Liao, Y., Kong, W. Z., & Wang, S. H. (2020). ATP dynamic regeneration strategy for enhancing co-production of glutathione and S-adenosylmethionine in Escherichia coli. Biotechnology Letters, 42(12), 2581–2587.