BPC-157 as Magical Synthetic Peptide
Abstract
BPC-157, also known as Body Protective Compound-157, is a synthetic peptide consisting of 15 amino acids derived from a naturally occurring protein. This intriguing compound has garnered significant interest in the fields of medical research and sports medicine due to its purported therapeutic effects.
Studies suggest that BPC-157 may possess the remarkable ability to promote healing and tissue repair, reduce inflammation, and offer protective benefits to the gastrointestinal tract. By interacting with various biological pathways involved in tissue regeneration and healing, BPC-157 has been investigated for its potential in treating a wide range of ailments, including musculoskeletal injuries, ulcers, and inflammatory bowel disease. Furthermore, some research indicates that it may accelerate the recovery process in tendons, ligaments, and bones. Its ability to facilitate angiogenesis, the formation of new blood vessels, has also raised interest in its potential applications for improving blood flow and wound healing. While the exact mechanisms through which BPC-157 exerts its effects are still under investigation, preliminary findings have shown promising results, generating enthusiasm within the scientific community.
However, it’s essential to approach the use of BPC-157 with caution, as more extensive research is necessary to fully understand its long-term safety and efficacy. As with any experimental compound, seeking guidance from qualified healthcare professionals and adhering to proper dosages are crucial steps for anyone considering exploring the potential benefits of BPC-157. The continuous advancement of research in this area may shed further light on BPC-157’s therapeutic properties, offering exciting possibilities for future medical treatments and improved healing mechanisms.
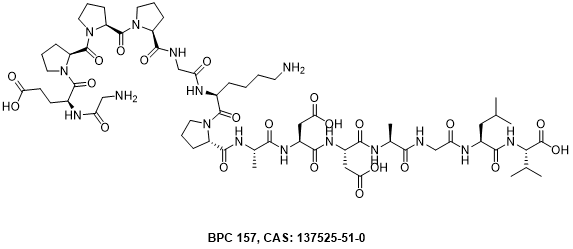
Figure 1 The structure of BPC 157
BPC 157 in stomach cell protection
In essence, the primary advantage of stomach cell protection in the cytoprotection concept lies in its direct origin in the stomach to counter mucosal lesions [1]. This unique feature allows for the quick resolution of damaged epithelial integrity [2]. While several agents, including standard anti-ulcer drugs, have been linked to stomach cell protection and cytoprotective effects [3], achieving a comprehensive generalization of the concept has proven challenging.
Attempts to generalize the stomach cell protection concept beyond the stomach’s innate cells to other organs have encountered theoretical and practical inconsistencies [4]. While prostaglandins were recognized for their protective effects in the stomach and intestine, their restricted action contrasts with the wider involvement of other epithelia, such as the skin and liver. Similarly, certain agents like somatostatin or sulphydrils were not acknowledged as organoprotective agents. Moreover, NSAIDs, known for their toxicity in various organs, remained limited to their damaging effect on the stomach mucosa.
A significant drawback within the stomach cell protection concept is its apparent limitation to prophylactic effectiveness with standard cytoprotective agents. These agents act only before exposure to noxious substances, lacking therapeutic potential when administered after injury. This limited prophylactic activity has become widely accepted as the essential characteristic of standard cytoprotective agents.
To bridge the gap between local damage to the stomach mucosa and the general toxicity seen in other organs caused by alcohol and NSAIDs, further research is needed. The ideal cytoprotective agent should be effective both before and after injury induction, exerting continuous innate cell protection. Current methodological and theoretical limitations should be addressed in future cytoprotective research to refine and enhance the concept.
BPC 157 proves to be a highly effective cytoprotective agent, resolving previous drawbacks associated with standard cytoprotective agents [5]. It exhibits authentic stability in human gastric juice for over 24 hours, continuously preserving gastrointestinal mucosal integrity [6]. Unlike other agents, BPC 157 is administered alone, attributing its beneficial effects unmistakably to its own activity [7], thereby qualifying as an organoprotective agent.
Remarkably, BPC 157 demonstrates an exceptional wound healing concept, promoting healing not only in gastrointestinal ulcers but also in deep skin burns, injured muscles, tendons, ligaments, bones, and nerves, as well as in tissues like the cornea [8]. Moreover, it exhibits a unique ability to heal fistulas and failed anastomoses, enabling the simultaneous healing of diverse tissues.
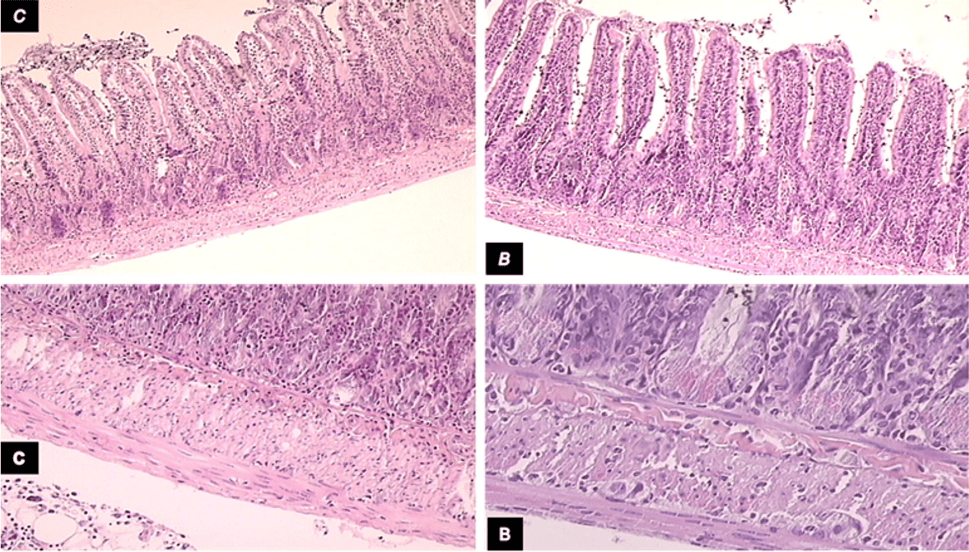
Figure 2 Microscopic presentation of intestinal adaptation in diclofenac-treated animals.
Microscopic presentation of intestinal adaptation in diclofenac-treated, controls (C), and BPC 157-treated (B) 24 h-short bowel rats. Upper left, control (C) shows lower mean villus height and shallower crypt depth, H&E, 10×); upper right, BPC 157 (B) shows increased villus height and crypt depth, H&E, 10×; lower panel, control (c) shows muscle thickness was less increased (H&E, 20×; and lower panel, BPC 157 (B) shows muscle thickness was increased more H&E, 20×)[8]
Regarding alcohol, BPC 157 acts as a potent antagonist, effectively counteracting both local and systemic effects induced by alcohol intoxication. This includes a full counteraction of alcohol intoxication, both acutely and chronically, indicating its active gut-brain axis or brain-gut axis functioning. In rats with chronic alcohol consumption, BPC 157 antagonizes gastric, liver, and lung lesions caused by alcohol.
Additionally, BPC 157 exhibits antidote activity against injuries induced by non-steroidal anti-inflammatory drugs (NSAIDs), countering lesions in the gastrointestinal tract, liver, brain, and bleeding disturbances. It effectively counteracts the inherent ulcerogenic potential of various NSAIDs and provides protection even in conditions of ethanol stomach lesions, somatosensory neurons depletion, short bowel, and NOS-blockade.
In conclusion, BPC 157 stands out as a powerful cytoprotective agent with a wide range of therapeutic benefits, demonstrating its potential as a valuable mediator in stomach cytoprotection and organoprotection.
BPC157 as Potential Agent Rescuing from Cancer Cachexia
Cancer cachexia, a debilitating clinical condition, is characterized by significant weight loss accompanied by specific losses of skeletal muscle and adipose tissue. It is driven by excessive energy expenditure, catabolism, and inflammation linked to increased proinflammatory cytokines, enhanced proteasomic muscle degradation, and significant lipolysis [9]. This condition disrupts energy balance, leads to drastic metabolic changes, and causes a notable decrease in fat mass and apparent skeletal muscle atrophy due to perturbing proinflammatory cytokines. While anorexia is often associated with cancer development, reduced food intake compounded by secondary nutrition derangement is thought to be the core initiator of cancer cachexia. However, concurrent hypermetabolism, hypercatabolism, and hypoanabolism provoked by systemic inflammation and catabolic factors, not reversed by conventional nutritional support, lead to progressive and irreversible impairment. Recent advancements in understanding cancer cachexia involve various mediators derived from cancer cells and the tumor microenvironment, including inflammatory and immune cells, endocrine and metabolic perturbations, and catabolic changes in skeletal muscle and adipose tissue. Despite current limitations in counteracting cancer cachexia due to its complex pathogenesis and lack of targeted agents, BPC157, a pentadecapeptide, shows promising potential as a fundamental agent targeting the signaling processes implicated in cancer cachexia. Its cytoprotective, angiogenic, regenerative, and anti-inflammatory properties make it a potential agent for mitigating cancer cachexia, especially due to its effectiveness in antagonizing key proinflammatory cytokines involved in the condition [10].
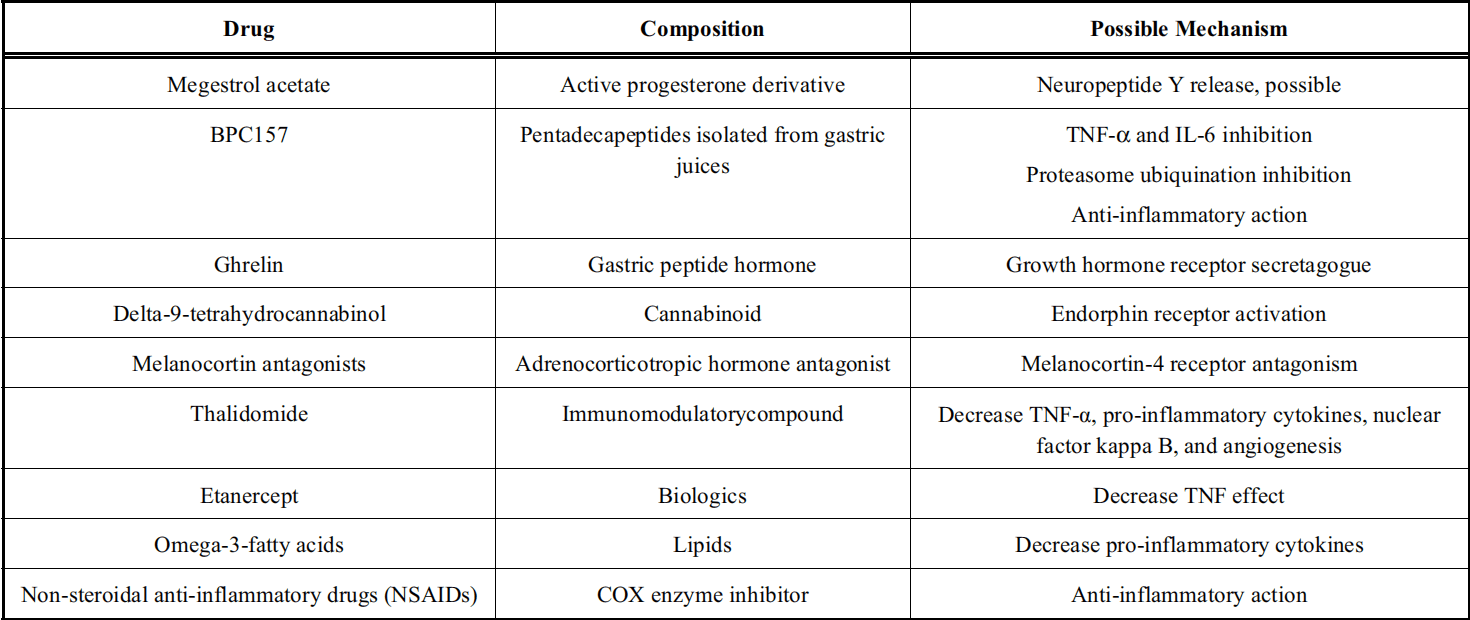
Figure 3 Potential future candidates for the treatment of cancer cachexia[11]
In this study[11], the researchers investigated the efficacy of BPC157 in a cancer cachexia model induced by C26 adenocarcinoma cells in Balb/c mice. The mice were injected with BPC157 three times per week, and the results were compared with a cancer cachexia control group. The administration of BPC157 showed a statistically significant improvement in halting weight loss compared to the control group. Importantly, BPC157 did not have any anti-cancer effects as evidenced by resecting xenograft tumors, which did not show any difference in tumor volumes or food intake between the groups. Instead, BPC157 appeared to significantly retard muscle atrophy induced by cancer cachexia. Observations of the whole thigh and leg muscles further supported the positive effects of BPC157 on muscle preservation. Muscle mass was significantly preserved in the BPC157-treated group, while the cancer cachexia group showed considerable muscle atrophy accompanied by muscle degeneration. Changes in the expression of muscle-related proteins, such as Atrogin-1, MuRF-1, and Mfn-2, were consistent with the muscle preservation effect of BPC157. BPC157 was shown to encourage muscle regeneration and inhibit muscle atrophy by affecting the expression of muscle-specific transcription factors like PAX-7 and PGC-1α. Inflammation plays a critical role in cancer cachexia, and BPC157 effectively suppressed the expressions of TNF-α and IL-6, both of which are pro-inflammatory cytokines implicated in cachexia. Furthermore, BPC157 positively influenced muscle metabolism, correcting deranged muscle proliferation and myogenesis, as shown by changes in FoxO3a, p-AKT, p-mTOR, and P-GSK-3β expression.
Collectively, the findings demonstrated that BPC157 has significant mitigating effects against cancer cachexia-induced muscle degeneration, inflammation, and catabolism. These results suggest that BPC157 could be a potential candidate for addressing the complex pathophysiology of cancer cachexia. However, further detailed clinical trials are warranted to explore its potential in rescuing patients or improving their quality of life. Overall, BPC157 shows promise as a novel therapeutic approach for combating the devastating effects of cancer cachexia.
Summary
BPC-157, also known as Body Protective Compound-157, is a synthetic peptide composed of 15 amino acids derived from a naturally occurring protein. This compound has drawn significant interest in medical research and sports medicine due to its potential therapeutic effects. Studies suggest that BPC-157 possesses unique healing properties, reduces inflammation, and offers protective benefits to the gastrointestinal tract. It has been investigated for its potential in treating various ailments, including musculoskeletal injuries, ulcers, and inflammatory bowel disease.
In stomach cell protection, BPC-157 offers distinct advantages compared to standard anti-ulcer drugs. Its direct origin in the stomach enables quick resolution of damaged epithelial integrity, making it an effective cytoprotective agent. BPC-157 demonstrates stability in human gastric juice, preserving gastrointestinal mucosal integrity continuously. It functions as an organoprotective agent, attributing its beneficial effects to its own activity rather than acting as a prophylactic agent. BPC-157 also shows an exceptional wound healing concept, promoting healing in various tissues, including deep skin burns, injured muscles, tendons, ligaments, bones, and nerves. Its antagonistic effects against alcohol and NSAID-induced injuries further enhance its potential as a cytoprotective agent.
Regarding cancer cachexia, BPC-157 exhibits potential as a fundamental agent targeting signaling processes implicated in the condition. Studies show that BPC-157 effectively retards muscle atrophy induced by cancer cachexia without any anti-cancer effects. It preserves muscle mass and encourages muscle regeneration through its positive influence on muscle metabolism. Additionally, BPC-157 suppresses the expression of pro-inflammatory cytokines involved in cancer cachexia, making it a promising candidate for mitigating this debilitating clinical condition.
In conclusion, BPC-157 shows significant promise as a versatile and effective therapeutic agent in stomach cell protection and cancer cachexia. Its ability to promote healing, reduce inflammation, and protect gastrointestinal integrity make it an intriguing compound in medical research. However, thorough investigation and clinical trials are crucial to fully understand its potential and ensure its safe and effective use. As the scientific community continues to explore BPC-157’s therapeutic properties, its applications may revolutionize medical treatments and enhance healing mechanisms in various ailments and conditions.
Reference
- Robert, A. (1985). Cytoprotection and adapted cytoprotection. In Peptic Ulcer Disease: Basic and Clinical Aspects: Proceedings of the Symposium Peptic Ulcer Today, 21–23 November 1984, at the Sophia Ziekenhuis, Zwolle, The Netherlands (pp. 297-316). Springer Netherlands.
- Robert, A. (1981). Current history of cytoprotection. Prostaglandins, 21, 89-96.
- Sikirić, P., Rotkvić, I., Miše, S., Križanac, Š., Gjuriš, V., Jukić, J., … & Anić, T. (1988). The influence of dopamine agonists and antagonists on indomethacin lesions in stomach and small intestine in rats. European journal of pharmacology, 158(1-2), 61-67.
- Sikiric, P., Seiwerth, S., Brcic, L., Sever, M., Klicek, R., Radic, B., … & Kolenc, D. (2010). Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Current pharmaceutical design, 16(10), 1224-1234.
- Sikiric, P., Seiwerth, S., Rucman, R., Drmic, D., Stupnisek, M., Kokot, A., … & Lovric Bencic, M. (2017). Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution?. Current Pharmaceutical Design, 23(27), 4012-4028.
- Seiwerth, S., Brcic, L., Batelja Vuletic, L., Kolenc, D., Aralica, G., Misic, M., … & Sikiric, P. (2014). BPC 157 and blood vessels. Current pharmaceutical design, 20(7), 1121-1125.
- Sikiric, P., Seiwerth, S., Rucman, R., Kolenc, D., Batelja Vuletic, L., Drmic, D., … & Vlainic, J. (2016). Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Current neuropharmacology, 14(8), 857-865.
- Fearon, K., Arends, J., & Baracos, V. (2013). Understanding the mechanisms and treatment options in cancer cachexia. Nature reviews Clinical oncology, 10(2), 90-99.
- Keremi, B., Lohinai, Z., Komora, P., Duhaj, S., Borsi, K., Jobbagy-Ovari, G., … & Varga, G. (2009). Antiinflammatory effect of BPC 157 on experimental periodontitis in rats. J Physiol Pharmacol, 60(Suppl 7), 115-122.
- Kang, E. A., Han, Y. M., An, J. M., Park, Y. J., Sikiric, P., Kim, D. H., … & Hahm, K. B. (2018). BPC157 as potential agent rescuing from cancer cachexia. Current pharmaceutical design, 24(18), 1947-1956.