Ceritinib (LDK378): A Breakthrough in ALK-Positive Lung Cancer Treatment
Abstract
Ceritinib (LDK378) is a second-generation ALK inhibitor that has significantly advanced the treatment of ALK-positive non-small cell lung cancer (NSCLC). By specifically targeting abnormal ALK fusion proteins, Ceritinib offers a personalized therapy option, especially for patients who have developed resistance to first-generation inhibitors like crizotinib. Clinical trials demonstrate its efficacy in improving progression-free survival and overall response rates, and its ability to penetrate the central nervous system addresses brain metastases common in advanced NSCLC. While side effects such as gastrointestinal disturbances require management, adjusted dosing strategies have enhanced patient tolerability. Ongoing research focuses on overcoming resistance mechanisms and optimizing treatment, underscoring Ceritinib’s pivotal role in targeted cancer therapy.
Introduction to Ceritinib and ALK-Positive Lung Cancer
About 85% of all instances of lung cancer worldwide are Non-Small Cell Lung Cancer (NSCLC), making it the most common kind of the disease. Adenocarcinoma, Squamous Cell Carcinoma, and Large Cell Carcinoma are subtypes of Non-Small Cell Lung Cancer. Lung cancer continues to be the most common cause of cancer-related fatalities worldwide, despite advancements in medical research. This is mostly because of the disease’s aggressiveness and late diagnosis.
The identification of the genetic alterations responsible for the advancement of NSCLC was a major advance in our understanding of the disease. The gene for anaplastic lymphoma kinase (ALK) is one example of such a mutation. When a segment of the ALK gene fuses with another gene, an aberrant ALK fusion protein is produced that encourages unchecked cell development, leading to ALK-positive NSCLC. About 3-7% of NSCLC patients have this genetic change, and these patients are typically younger, nonsmokers or mild smokers.
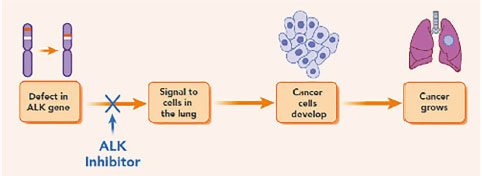
Fig.1 An overview of ALK-positive non-small cell lung cancer
Treatment choices for individuals with ALK-positive NSCLC have been completely transformed by the development of targeted medicines. Second-generation ALK inhibitor crizitinib (LDK378) was created to overcome resistance to crizotinib and other first-generation inhibitors. Ceritinib, which was approved by the FDA in the United States in 2014, has been effective in treating patients who have become intolerant to crizotinib or have progressed while on it. It functions by specifically blocking the action of the ALK fusion protein, which stops the growth of cancer cells and causes tumor regression. Ceritinib treatment has significantly improved overall response rates and progression-free survival, according to clinical trials.
A significant development in lung cancer patients‘ customized therapy is the launch of Ceritinib. Compared to conventional chemotherapy, therapies like Ceritinib provide better results and a higher quality of life by focusing on particular genetic abnormalities. Its full potential is still being investigated, including possible combinations with other treatments to increase efficacy and get around resistance mechanisms.
Mechanism of Action of Ceritinib
A powerful and specific second-generation inhibitor, cetinib targets the tyrosine kinase receptor of anaplastic lymphoma kinase (ALK), which is linked to the etiology of a fraction of non-small cell lung malignancies (NSCLC). Chromosome rearrangements in ALK-positive NSCLC result in fusion genes that generate constitutively active ALK fusion proteins, which promote tumor growth and survival.
How Ceritinib Works as an ALK Inhibitor
Ceritinib inhibits the kinase activity of ALK by attaching itself to the ATP-binding site of the kinase domain. The PI3K/AKT and RAS/MAPK pathways, which are essential for cell division, survival, and metastasis, are blocked by this inhibition. Ceritinib causes tumor cell growth inhibition and apoptosis by interfering with certain signaling cascades.
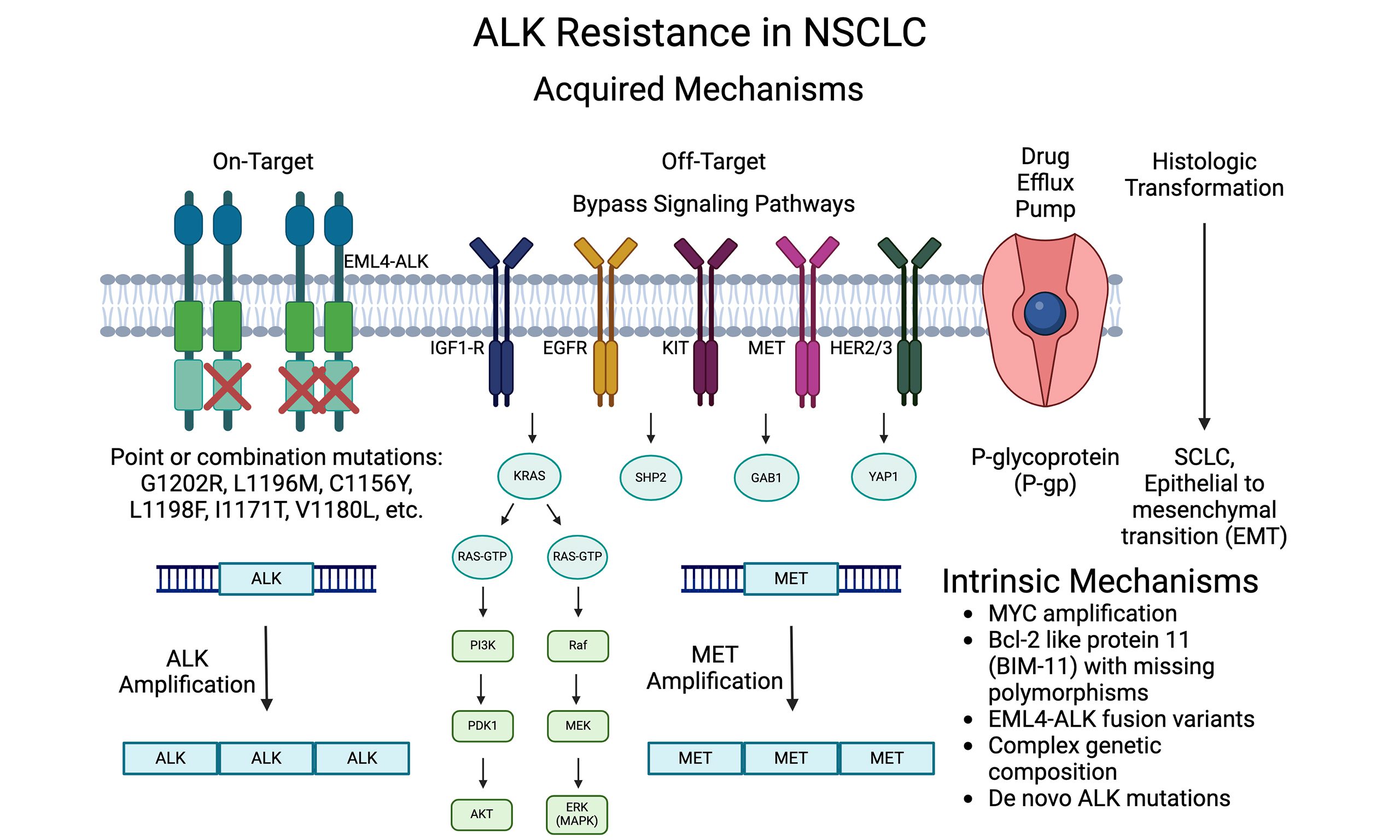
Fig.2 ALK inhibitors in cancer
Advantages Over First-Generation ALK Inhibitors
Ceritinib is more potent and effective than first-generation ALK inhibitors such as crizotinib, particularly in beating resistance mutations. L1196M and G1269A are two secondary mutations in the ALK kinase domain that frequently lead to crizotinib resistance. Patients who have grown resistant to crizotinib now have a useful therapeutic option in crizotinib, which has shown efficacy against these mutations. It’s a breakthrough in targeted cancer therapy since it can target a wider range of ALK mutations.
Impact on Cancer Cell Growth and Spread
Ceritinib decreases the potential for metastasis and angiogenesis while simultaneously suppressing the growth of tumor cells by the efficient inhibition of ALK-mediated signaling pathways. Clinical research has demonstrated that in patients with ALK-positive NSCLC, crizitinib significantly reduces tumor size and extends progression-free survival. Furthermore, Ceritinib has proven to be able to enter the central nervous system, which means that patients who have brain metastases—a typical side effect of advanced lung cancer—can benefit from treatment.
Clinical Applications and Efficacy
Indications for Ceritinib Use
Patients with metastatic non-small cell lung cancer (NSCLC) that is positive for anaplastic lymphoma kinase (ALK) should be treated with crizitinib. It is especially prescribed for patients who have become resistant to crizotinib, a first-generation ALK inhibitor, or who have progressed on it. Ceritinib is sometimes licensed for use as an ALK-positive NSCLC first-line treatment option, providing newly diagnosed patients with a useful substitute.
Dosage and Administration Guidelines
In order to increase patient tolerance, Ceritinib dosage recommendations have changed. Studies found that the initial recommended dosage of 750 mg, which was to be taken orally once day on an empty stomach, caused substantial gastrointestinal adverse effects. In order to improve tolerance, the dosage was changed to 450 mg once daily, taken with food. This adjustment has decreased side effects without sacrificing therapeutic efficacy. It is recommended that patients take Ceritinib with food at the same time every day, and that they swallow the capsules whole, without crushing or chewing. Depending on each person’s tolerance and the occurrence of side effects, dose modifications can be required.
Results from Key Clinical Trials
Ceritinib has proven to be effective in treating ALK-positive non-small cell lung cancer in clinical trials. Patients who had previously received crizotinib and chemotherapy showed an overall response rate (ORR) of 56% in the ASCEND-1 trial, indicating a significant reduction in tumor size. Ceritinib and platinum-based chemotherapy were compared in treatment-naïve patients in the ASCEND-4 trial. According to the study, progression-free survival (PFS) was considerably extended by crizitinib, with a median PFS of 16.6 months as opposed to 8.1 months for chemotherapy. Ceritinib has also demonstrated intracranial effectiveness in patients with brain metastases, a frequent side effect in advanced non-small cell lung cancer, thus filling a vital need in this patient group. These findings highlight the significance of crizinib as a treatment option that can significantly improve prognoses for patients with NSCLC that is positive for ALK.
Side Effects and Safety Considerations
Common Side Effects
Similar to other targeted cancer treatments, crizitinib is linked to a number of side effects that patients may encounter while undergoing treatment. The gastrointestinal side symptoms—diarrhea, nausea, vomiting, and abdominal pain—are the most commonly reported side effects. Ceritinib’s impact on the gastrointestinal tract’s quickly dividing cells causes these symptoms. Other common adverse effects that patients may encounter include fatigue, decreased appetite, and weight loss.
Serious Adverse Effects
Healthcare professionals must closely monitor more severe side effects. With some patients exhibiting higher liver transaminases, suggesting possible liver injury, hepatotoxicity is a serious concern. It is advised to have routine liver function testing to quickly identify and manage this risk. Pneumonitis, also known as interstitial lung disease, is less prevalent but can still be fatal. Its symptoms include coughing and dyspnea. Prolongation of the QT interval is another dangerous side effect that can cause arrhythmias and interfere with cardiac rhythm. In order to detect any cardiac problems early in the course of treatment, electrocardiogram monitoring is indicated.
Monitoring and Managing Side Effects
Sustaining quality of life and ensuring therapy continuation need effective control of side effects. Depending on the intensity of adverse effects, dose modifications, such as decreases or brief stops, can be required. Discomfort can be reduced with supportive care interventions such antiemetic drugs for nausea and dietary changes for gastrointestinal problems. It is recommended that patients rapidly notify their healthcare staff of any new or worsening symptoms. The overall safety profile of Ceritinib therapy is improved by rapid discovery and control of adverse effects made possible by routine monitoring through laboratory testing and clinical evaluations.
Future Directions and Research
Ongoing Studies and Potential Enhancements
The effectiveness and safety of crizitinib in the treatment of ALK-positive non-small cell lung cancer (NSCLC) are still being investigated. Clinical trials are looking into the best ways to dose patients, such as taking smaller dosages with food to lessen gastrointestinal adverse effects without sacrificing the effectiveness of the medication. In order to enhance patient outcomes, studies are also looking into the use of Ceritinib in early stages of NSCLC and in conjunction with other medicines.
Ceritinib in the Landscape of Targeted Therapies
One major difficulty that still exists with ALK inhibitors is the development of resistance. Understanding the mechanisms of Ceritinib resistance and creating next-generation ALK inhibitors that can get beyond these obstacles are the main goals of research. Combination treatments that focus on several pathways are being researched to stop the emergence of resistance and offer longer-lasting effects.
Personalized Medicine and Genetic Profiling
Novel developments in biomarker discovery and genetic profiling are opening the door to personalized medical strategies. Healthcare professionals can choose the best therapy for each patient by customizing treatments based on unique genetic mutations and tumor features. Real-time monitoring of disease progression and therapy response is being pursued through the development of liquid biopsies and other non-invasive testing techniques.
Potential Impact on Other Cancers
The effectiveness of crizitinib in treating various tumors with ALK mutations or rearrangements is also being studied. Ceritinib may have advantages in treating malignancies other than non-small cell lung cancer, including inflammatory myofibroblastic tumors and anaplastic large cell lymphoma, according to preliminary research.
Conclusion
Patients who had few therapy choices in the past for ALK-positive non-small cell lung cancer (NSCLC) now have hope thanks to the introduction of ceritinib (LDK378). Ceritinib offers a customized strategy that has shown significant effectiveness in clinical settings by precisely targeting the abnormal ALK fusion proteins that drive tumor growth and proliferation. Its capacity to overcome resistance to ALK inhibitors of the first generation, such as crizotinib, represents a significant breakthrough in the treatment of this subset of patients with lung cancer.
Ceritinib has demonstrated in clinical trials its ability to improve overall response rates and progression-free survival in patients who have developed resistance to previous medications as well as in patients receiving it as a first-line treatment. Furthermore, since brain metastases are frequent in advanced non-small cell lung cancer, its ability to enter the central nervous system meets a vital demand. These advantages highlight the value of further research and development in targeted medicines and highlight the contribution that personalized medicine can make to improving patient outcomes.
Ceritinib is a huge advancement, but controlling its side effects is still necessary to maximize treatment compliance and patient quality of life. Healthcare professionals must strike a balance between effectiveness and acceptability, changing dosages as needed and provide supportive care to lessen side effects. Reducing toxicity and overcoming resistance mechanisms may be possible with further attempts to explore combination medicines and improve dosage regimens.
Looking ahead, more innovation and customisation will be key components of the therapy of ALK-positive NSCLC. More accurate targeting of cancer cells will be possible thanks to developments in genetic profiling and biomarker identification, which may result in the creation of ALK inhibitors with better safety and effectiveness profiles in the future. Working together, patients, doctors, and researchers can propel these developments toward the ultimate goal of turning ALK-positive NSCLC into a chronic illness that can be controlled.
References
- Shaw, A. T., Kim, D. W., Mehra, R., Tan, D. S. W., Felip, E., Chow, L. Q., … & Engelman, J. A. (2014). Ceritinib in ALK-rearranged non–small-cell lung cancer. New England Journal of Medicine, 370(13), 1189–1197.
- Soria, J. C., Tan, D. S., Chiari, R., Wu, Y. L., Paz-Ares, L., Wolf, J., … & de Marinis, F. (2017). First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. The Lancet, 389(10072), 917–929.
- Friboulet, L., Li, N., Katayama, R., Lee, C. C., Gainor, J. F., Crystal, A. S., … & Engelman, J. A. (2014). The ALK inhibitor ceritinib overcomes crizotinib resistance in non–small cell lung cancer. Cancer Discovery, 4(6), 662-673.
- Katayama, R., Shaw, A. T., Khan, T. M., Mino-Kenudson, M., Solomon, B. J., Halmos, B., … & Engelman, J. A. (2012). Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Science Translational Medicine, 4(120), 120ra17.
- Cho, B. C., Kim, D. W., Bearz, A., Laurie, S. A., McKeage, M., Borra, G., … & Kim, S. W. (2017). ASCEND-8: A randomized phase I study of Ceritinib 450 mg or 600 mg taken with a low-fat meal versus 750 mg fasted in patients with ALK-rearranged metastatic NSCLC. Journal of Thoracic Oncology, 12(9), 1357–1367.
- Crinò, L., Ahn, M. J., De Marinis, F., Groen, H. J., Wakelee, H., Hida, T., … & Felip, E. (2016). Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non–small-cell lung cancer previously treated with chemotherapy and crizotinib: Results from ASCEND-2. Journal of Clinical Oncology, 34(24), 2866–2873.
- Gainor, J. F., & Shaw, A. T. (2014). Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. Journal of Clinical Oncology, 31(31), 3987–3996.
- Scagliotti, G., Brodowicz, T., Shepherd, F. A., Zielinski, C., Vansteenkiste, J., Manegold, C., … & Mellemgaard, A. (2017). Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non–small cell lung cancer. Journal of Thoracic Oncology, 6(1), 64–70.




