Challenges and Opportunities in Cancer Models of Aging
Abstract
Age is one of the major risk factors for cancer, and most cancer cases are diagnosed in people over the age of 65, with 60% of new cancer diagnoses and 70% of cancer deaths occurring in this population. Any cancer research on adults faces the problem of tissue and organism aging. However, most current preclinical studies of cancer are conducted using young animals. Therefore, they cannot directly address the impact of aging on various aspects of cancer occurrence, development, heterogeneity, and treatment response. The underrepresentation of patients over the age of 65 in clinical trials also illustrates the disconnect between aging and cancer research. This is a problem because the elderly exhibit comorbidities that may affect drug efficacy and toxicity, which cannot be fully reflected in young people. Therefore, in a large proportion of cancer patients, key issues related to tumorigenesis, treatment efficacy, and drug resistance have not been fully resolved. Therefore, the construction and validation of new cancer models are essential to accelerate preclinical discovery and translation.
Characteristics of aging to consider in cancer model development
Aging is a complex biological and physiological process accompanied by significant changes in the cellular and molecular components of tissues and organ systems. Therefore, the choice of model system should depend on the aspects of aging one wishes to capture, such as biological aging, immunosenescence (local vs. systemic), inflammation, or aging and senescence of the microenvironment.
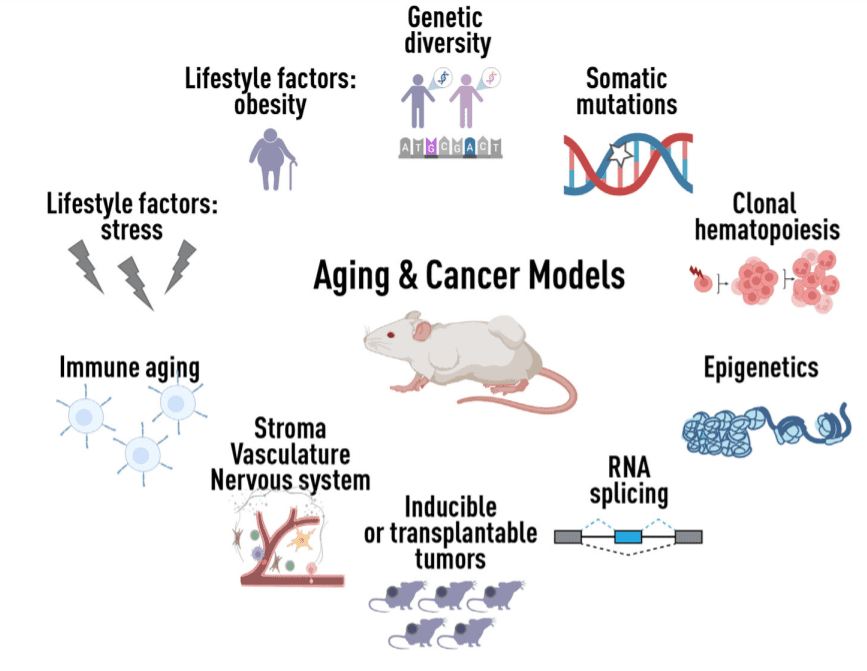
Figure 1 Developing new models for age-related cancers
At the cellular level, aging is characterized by mitochondrial dysfunction and altered metabolic programs. At the molecular level, aging tissues exhibit genomic changes, including the accumulation of somatic mutations, as well as epigenetic, transcriptional, and post-transcriptional remodeling. At the systemic level, aging is often accompanied by cognitive dysfunction, muscle and bone loss, functional changes at hormonal and endocrine levels, immune dysfunction, and chronic inflammation.
The complex role of aging in cancer requires careful dissection in the context of model systems. It is known that senescence-induced changes in the microenvironment of distant metastasis promote the efficient activation of dormant cancer cells. A fundamental unanswered question is how these biological features of aging influence cancer initiation, progression, and treatment response. Furthermore, these characteristics and the diseases of aging they cause do not exist in isolation but in combination, they can influence each other and they can exhibit heterogeneity within and between individuals.
The design of in vivo models requires understanding not only the effects of age, but also the effects of biological sex, hormonal signaling, genetic background, immune status, diet, physical activity, exposure to infectious agents, environment, and inflammation. Furthermore, there is a need to better incorporate possible additive or synergistic effects of other aging-related comorbidities detected in cancer patients.
Modeling mutations and genetic alterations
Aged cancer models should allow us to dissect which cellular and molecular components have the greatest impact on the cell of origin of cancer, the fitness of the premalignant cells that initiate it, and the mechanisms of tumor progression and metastasis. Most mouse tumor models, including some of the classic models that discovered key oncogenes or tumor suppressors (e.g., Trp53, Brca1, Her2), do not accurately reflect or recapitulate the cellular or molecular environment of the elderly or genetically diverse populations in which tumors arise. Establishing inducible models that activate oncogenes or inactivate tumor suppressors in aged animals in a tissue-specific manner will enable monitoring of how these changes alter epithelial, immune, and stromal components with age.
Aged cancer models should also incorporate somatic mutations, which accumulate over the life of an organism and can affect host function. This phenomenon is best illustrated by clonal hematopoiesis, where mutant clones have a fitness advantage and contribute to the generation of a significant proportion of mature blood cells. Mutations in genes involved in epigenetic regulation (DNMT3A, TET2, ASXL1) account for the majority of mutation-driven clonal hematopoiesis in humans. These mutations are rare in young people but very common in the elderly, with 10%-20% of people over 70 years old harboring sizable clones. Clonal hematopoiesis has been shown to transform to acute myeloid leukemia in vivo by inducing additional cooperating leukemogenic driver mutations. The effects of clonal hematopoiesis on myeloid cells may lead to myeloid dysfunction in tissues even in the absence of leukemic transformation.
Finally, multiple studies describe the age-related accumulation of cells in phenotypically normal human tissues that harbor mutations that are often classified as oncogenic, some of which are even present from birth. However, these are often insufficient to drive tumorigenesis alone. Therefore, models that can measure aging-associated changes in the fitness of cells carrying tumor-associated mutations are needed.
Simulating immunosenescence
The decline of the immune system as we age is reflected in increased susceptibility to infectious diseases, poorer responses to vaccinations, and increased rates of cancer, autoimmune disease, and other chronic diseases. Aging is associated with significant changes in innate and adaptive immunity. For example, hematopoiesis is tilted toward myelopoiesis, whereas lymphopoiesis decreases with age. In the periphery, T cells undergo major age-related changes, including a decrease in naive T cells due to thymic involution and an increase in the number of terminally differentiated memory T cells and exhausted T cells. Similar phenotypic and functional changes have been observed in myeloid cells, including dendritic cells and macrophages.
Similar age-related changes in immune populations have been observed in some mouse strains commonly used in cancer research. Therefore, cancer models that rely on young inbred animals do not accurately reflect the immune microenvironment of tumorigenesis in older patients, but some mouse strains may be suitable for studying “immune age.” In fact, neither humans nor mice need to be elderly to exhibit an immunosenescent phenotype. Results from cancer studies may be confounded by the use of older mice, where morbidity is associated with morbidity later in the mouse’s lifespan, necessitating the development of new models driven by specific questions.
Additionally, another key limitation of many current mouse models of aging cancer is that they are kept under sterile conditions and are not exposed to, for example, diet, carcinogens, viruses, bacteria, or vaccines that stimulate the immune system throughout the human lifetime. External triggers. Chronic exposure to pro-inflammatory environmental factors is a possible mechanism for accelerated aging and cancer development. This concept could be rigorously tested in mouse models, for example, by chronic activation of the immune system through vaccination or exposure to inflammation.
Modeling therapeutic responses
Aged cancer models will be highly valuable for dissecting how aging affects responses to different cancer treatments, as well as determining whether therapeutic drugs or combinations are more effective in older animals and associated with reduced side effects. For example, while most cancer patients are still receiving chemotherapy, dosing, pharmacokinetics, and pharmacodynamics in older patients remain poorly understood because most compounds are tested in clinical trials in younger patients. Similarly, the efficacy of immunotherapy in patients with aged immune systems remains unclear.
In addition, it will be necessary to define disease responses in different age groups. For example, recent molecular characterization of human cancers has shown that some adult cancers look very different in young and old people, such as triple-negative breast cancer. As another example, there is a difference in response to VEGF-targeted therapy in young versus old melanoma patients, with the latter seeing little benefit. Age-stratified responses to treatment appear to differ across cancer types, and therefore, cancer models that test age-stratified treatments will be very useful and clinically relevant.
Modeling Host Genetic Diversity
Another major challenge in aging cancer models is how to robustly model the age-related complexity and heterogeneity of cancer biology in genetically identical inbred mice. Cancer is a phenomenon that relies heavily on genetics, and the biological complexity of aging in cancer research will further require addressing genetic diversity in model systems.
The Hybrid Mouse Diversity Panel (HMDP) is a powerful platform to model human genetic diversity and determine how individual genetic variation contributes to the complexity of aging and cancer. By using aged, genetically diverse collaborative cross (CC)/diverse outbred (DO) mice that exhibit a wide range of phenotypic variation in tumor susceptibility, we may gain new insights into how genetic background affects aging-related cellular processes and potentially identify common biochemical pathways between mice and humans. For example, DO mice can be used for phenotypic, epigenetic, and transcriptomic analysis of various tissues in a study that examined different ages (e.g., 12, 18, and 24 months) while performing comprehensive histopathological assessments to identify pre-neoplastic or neoplastic lesions.
Genotypically diverse mice can further be used to combine studies of acute and chronic inflammation or immune stimulation. Analysis of aging-associated changes in host and cancer behavior, along with multi-omics data comparable to humans, will help us select the most representative strains and models of human diversity to study specific cancer behaviors.
Summary
Given that cancer is primarily a disease of the elderly, a better understanding of the interplay between aging and cancer will help improve cancer outcomes in older patients. Therefore, studies combining cancer and aging mouse models are essential. Future expansion of the cross-species comprehensive analysis toolkit will help us identify and ideally explain these differences. The successful development of clinically relevant models of age-related cancers will allow us to more effectively test therapeutic interventions and ultimately develop preventive strategies.
Reference




