Cobenfy: Novel Antipsychotic Targeting Muscarinic Receptors for Schizophrenia Treatment
Abstract
Cobenfy (xanomeline and trospium chloride) is a newly FDA-approved antipsychotic medication that targets muscarinic receptors, marking a significant shift from traditional dopamine-based treatments for schizophrenia. Clinical trials have demonstrated its ability to effectively reduce both positive and negative symptoms of schizophrenia, with fewer metabolic side effects than existing antipsychotics. The drug’s dual mechanism, combining xanomeline for symptom control and trospium chloride to minimize gastrointestinal effects, offers improved patient tolerance. Ongoing research explores Cobenfy’s potential use in treating conditions such as Alzheimer’s disease-related psychosis and Bipolar I disorder, further expanding its therapeutic applications. With its unique approach and promising safety profile, Cobenfy is positioned as a groundbreaking option for managing schizophrenia and other neurological disorders.
Introduction: A New Era in Schizophrenia Treatment
Cobenfy (xanomeline and trospium chloride) represents a breakthrough in schizophrenia treatment, marking the first approval of an antipsychotic drug targeting muscarinic receptors instead of traditional dopamine pathways. Schizophrenia, a severe and chronic mental illness, affects approximately 1% of the global population, and existing treatments often rely on dopamine receptor inhibition. However, these treatments frequently lead to side effects like weight gain, metabolic issues, and movement disorders. Cobenfy offers a fresh alternative by focusing on the muscarinic receptors M1 and M4, which are involved in regulating neurotransmission and cognitive functions.
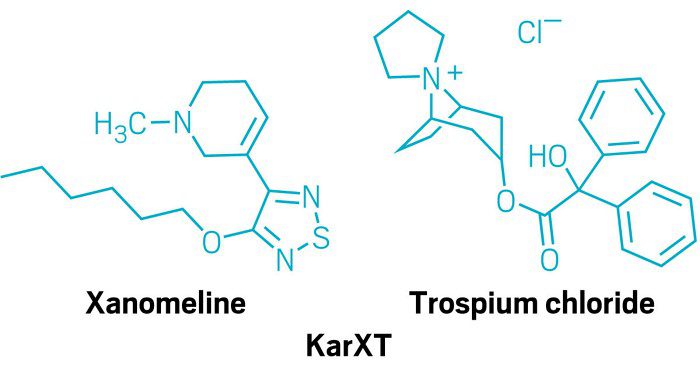
Fig.1 KarXT, a drug to treat schizophrenia, consists of xanomeline and trospium.
What sets Cobenfy apart is its ability to alleviate both positive and negative symptoms of schizophrenia, such as hallucinations, social withdrawal, and cognitive deficits, areas where dopamine-based treatments often fall short. Clinical trials demonstrated significant improvements in schizophrenia symptoms using Cobenfy, without the severe metabolic side effects commonly seen in older antipsychotics.
Additionally, the dual composition of Cobenfy—xanomeline to manage symptoms and trospium chloride to mitigate side effects—improves patient tolerance and overall safety. As the first antipsychotic in decades to introduce a novel mechanism of action, Cobenfy represents a promising new chapter in treating schizophrenia, offering hope to patients who have struggled with traditional therapies.
Mechanism of Action: Targeting Muscarinic Receptors
Cobenfy (xanomeline and trospium chloride) introduces a novel mechanism for managing schizophrenia by targeting the M1 and M4 muscarinic receptors, rather than the traditional dopamine receptors used by most antipsychotic medications. This unique approach aims to address both the positive and negative symptoms of schizophrenia, including hallucinations, delusions, social withdrawal, and cognitive deficits. Unlike dopamine-based treatments, which can lead to metabolic side effects and movement disorders, Cobenfy offers a pathway that bypasses these issues while still effectively managing symptoms.
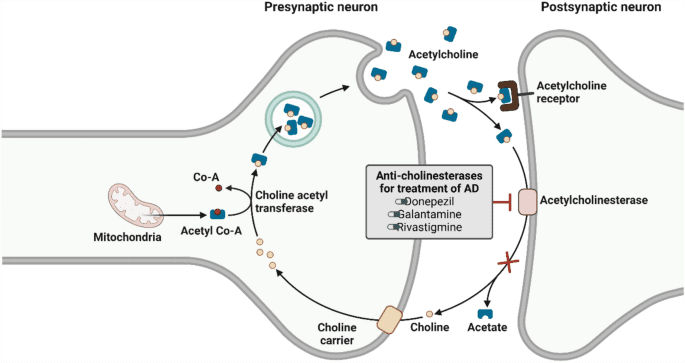
Fig.2 Current Findings and Potential Mechanisms of KarXT
Xanomeline, one of the active ingredients, interacts with muscarinic receptors in the central nervous system to modulate neurotransmission. This helps control schizophrenia symptoms without directly affecting dopamine receptors, which are traditionally linked to side effects like weight gain and involuntary movements. The second component, trospium chloride, is included to counteract the gastrointestinal side effects commonly associated with xanomeline, making Cobenfy more tolerable for patients.
By addressing both muscarinic and dopamine pathways indirectly, Cobenfy offers an innovative option for patients who have not responded well to existing treatments. This dual mechanism makes it particularly promising for reducing both psychotic and cognitive symptoms of schizophrenia, offering a more balanced approach to the disease’s complex neurobiology.
Clinical Trials: Efficacy and Safety
Cobenfy (xanomeline and trospium chloride) demonstrated promising efficacy and safety in clinical trials, leading to its approval as a treatment for schizophrenia. Two pivotal trials, EMERGENT-2 and EMERGENT-3, were conducted to evaluate the drug’s effectiveness in managing both positive and negative symptoms of schizophrenia. These were randomized, double-blind, placebo-controlled trials involving patients diagnosed with schizophrenia according to DSM-5 criteria.
In both trials, Cobenfy showed statistically significant improvements in schizophrenia symptoms as measured by the Positive and Negative Syndrome Scale (PANSS). The PANSS scores of patients receiving Cobenfy were substantially reduced by the end of the five-week trial compared to those receiving placebo. The trials also demonstrated improvements in cognitive symptoms, which are often difficult to treat with traditional antipsychotics.
Regarding safety, the most common side effects reported in the trials included nausea, constipation, and hypertension. Other side effects, such as gastrointestinal distress, were mitigated by the addition of trospium chloride, which helps reduce the gastrointestinal side effects often associated with xanomeline. Serious adverse effects, including angioedema and urinary retention, were rare but noted, underscoring the importance of monitoring patients during treatment.
The success of these trials highlights Cobenfy as a promising option for schizophrenia patients, especially those who have not responded well to traditional antipsychotics. With its unique mechanism targeting muscarinic receptors, Cobenfy offers both effective symptom control and a relatively favorable safety profile.
Comparison to Existing Antipsychotics
Cobenfy (xanomeline and trospium chloride) stands out from traditional antipsychotics due to its novel mechanism of action targeting muscarinic receptors rather than dopamine receptors, which most antipsychotic medications rely on. Traditional antipsychotics, such as clozapine and risperidone, often effectively reduce the positive symptoms of schizophrenia, like hallucinations and delusions, but they tend to be less effective at managing negative symptoms like social withdrawal and cognitive deficits. Moreover, these drugs come with a range of metabolic side effects, including weight gain, diabetes, and movement disorders.
In contrast, Cobenfy has been shown to improve both positive and negative symptoms, offering a more comprehensive approach to schizophrenia treatment. Additionally, it has been associated with fewer metabolic side effects, which is a significant advantage for patients who are concerned about weight gain and other long-term health risks. This makes it particularly appealing to patients who have experienced severe side effects with traditional antipsychotic drugs or who have not responded well to dopamine-targeting medications.
Another key advantage of Cobenfy is that it does not come with a boxed warning, which is often found on other antipsychotics due to risks such as increased mortality in elderly patients with dementia-related psychosis. This enhances its safety profile compared to older medications.
With its unique ability to treat both positive and negative symptoms, while minimizing metabolic side effects, Cobenfy offers a new and innovative option for managing schizophrenia.
Future Potential: Expanding Use Cases
Beyond its primary indication for schizophrenia, ongoing research into Cobenfy (xanomeline and trospium chloride) is exploring its potential for treating other neurological and psychiatric conditions. One of the most promising avenues of investigation is its use in managing Alzheimer’s disease-related psychosis. Alzheimer’s patients often experience agitation and psychotic symptoms, for which there are currently limited treatment options. Cobenfy’s unique mechanism, which avoids dopamine receptor interactions, positions it as a potential therapy for these symptoms without the significant metabolic or movement-related side effects of existing treatments.
In addition to Alzheimer’s, researchers are investigating the drug’s application in Bipolar I disorder, particularly for patients who exhibit psychotic features. This potential expansion into bipolar disorder treatment could offer a novel therapeutic option, especially for those who do not respond well to traditional antipsychotics.
Furthermore, early results from extended clinical trials suggest that Cobenfy may have long-term benefits without the severe side effects seen in other antipsychotics. While more data is needed to confirm its efficacy in these broader contexts, Cobenfy’s mechanism of action provides a strong foundation for future research in neurological and psychiatric disorders.
As these studies continue, Cobenfy may not only revolutionize schizophrenia treatment but also offer hope for patients suffering from other challenging conditions.
References
- Azargoonjahromi, A. (2024). Current findings and potential mechanisms of KarXT (Xanomeline-Trospium) in schizophrenia treatment. Clin Drug Investig, 44(7), 471-493. doi: 10.1007/s40261-024-01377-9. PMID: 38904739.
- Dudzik, P., Lustyk, K., & Pytka, K. (2024). Beyond dopamine: Novel strategies for schizophrenia treatment. Med Res Rev, 44(5), 2307-2330. doi: 10.1002/med.22042. PMID: 38653551.
- Howes, O. D., Dawkins, E., Lobo, M. C., Kaar, S. J., & Beck, K. (2024). New drug treatments for schizophrenia: A review of approaches to target circuit dysfunction. Biol Psychiatry, 96(8), 638-650. doi: 10.1016/j.biopsych.2024.05.014. PMID: 38815885.
- Paul, S. M., Yohn, S. E., Brannan, S. K., Neugebauer, N. M., & Breier, A. (2024). Muscarinic receptor activators as novel treatments for schizophrenia. Biol Psychiatry, 96(8), 627-637. doi: 10.1016/j.biopsych.2024.03.014. PMID: 38537670.
- Vasiliu, O., Budeanu, B., & Cătănescu, M.Ș. (2024). The new horizon of antipsychotics beyond the classic dopaminergic hypothesis-The case of the Xanomeline-Trospium combination: A systematic review. Pharmaceuticals (Basel), 17(5), 610. doi: 10.3390/ph17050610. PMID: 38794180; PMCID: PMC11124398.
- DeBattista, C., & Schatzberg, A. F. (2024). The Black Book of Psychotropic Dosing and Monitoring. Psychopharmacol Bull, 54(3), 8-59. PMID: 38993656; PMCID: PMC11235576.
- Ye, N., Wang, Q., Li, Y., & Zhen, X. (2024). Current emerging therapeutic targets and clinical investigational agents for schizophrenia: Challenges and opportunities. Med Res Rev. doi: 10.1002/med.22086. PMID: 39300769.
- Correll, C. U., Tusconi, M., Carta, M. G., & Dursun, S. M. (2024). What remains to be discovered in schizophrenia therapeutics: Contributions by advancing the molecular mechanisms of drugs for psychosis and schizophrenia. Biomolecules, 14(8), 906. doi: 10.3390/biom14080906. PMID: 39199294; PMCID: PMC11353083.




