Combating Antibiotic Resistance: Innovative Biochemical Strategies and the Role of Hydrogen Sulfide
Abstract
Antibiotic resistance is a pressing global health challenge, characterized by the ability of bacteria to withstand the effects of medications designed to combat infections. This phenomenon complicates treatment options for common diseases, raises healthcare costs, and increases morbidity and mortality rates. The World Health Organization has identified antibiotic resistance as a top public health threat, warning that without urgent intervention, millions of lives could be at risk annually. The emergence of drug-resistant strains, particularly in healthcare settings, underscores the need for innovative strategies to enhance the efficacy of existing antibiotics. Recent research has focused on biochemical approaches to counteract resistance mechanisms, including the SOS response and the role of hydrogen sulfide (H₂S) in bacterial tolerance. Understanding how H₂S production influences antibiotic susceptibility could lead to new therapeutic pathways that restore the effectiveness of current antibiotics. This review highlights key strategies such as immune-antibiotics, the inhibition of bacterial resistance pathways, and the development of H₂S-releasing compounds to address the growing crisis of antibiotic resistance.
Introduction
Antibiotics are crucial medications used to treat and prevent bacterial infections. However, antibiotic resistance arises when bacteria adapt due to the use of these drugs. This evolution leads to bacteria that no longer respond to treatment, complicating infection management and increasing the risks of disease transmission, severe illness, and death. Bacterial resistance denotes a microorganism’s ability to survive despite the presence of antibiotics at clinically relevant concentrations.The issue of antibiotic resistance is escalating globally, with the World Health Organization (WHO) warning that we are “running out of antibiotics.” This situation has intensified concerns over rising drug-resistant bacteria, which pose significant challenges in treating clinical infections and contribute to an increase in hospital-acquired infections. Bacterial infections remain a leading cause of illness and death worldwide, with new resistance mechanisms frequently emerging and spreading, threatening the treatment of common infectious diseases.
As antibiotics lose effectiveness, conditions like pneumonia, tuberculosis, blood poisoning, gonorrhea, and foodborne illnesses become increasingly difficult, if not impossible, to treat. We are approaching a post-antibiotic era where even minor injuries could prove fatal without urgent action. Sir Alexander Fleming discovered penicillin in 1929, heralded as a “wonder drug” for its effectiveness against infections. Yet, by the 1940s, resistance to penicillin had emerged, and currently, over 95% of Staphylococcus aureus strains are resistant.In response, methicillin was developed but was soon followed by the emergence of methicillin-resistant Staphylococcus aureus (MRSA). The introduction of broad-spectrum antibiotics in the late 1940s was initially effective, but the WHO now lists antimicrobial resistance among the top global public health threats. Projections suggest antibiotic-resistant infections could claim up to 10 million lives annually by 2050, costing the global economy approximately $100 trillion.More than 70% of pathogenic bacteria are believed to resist at least one available antibiotic. The rise of multidrug-resistant (MDR) bacteria is accelerated by the misuse of antibiotics in both healthcare and agriculture. The COVID-19 pandemic has exacerbated this issue, with studies indicating high rates of antibiotic prescriptions among COVID-19 patients, often without clinical justification, further fostering antibiotic resistance.
It is pertinent to state that the problem of antimicrobial resistance is aggravated by the lack of interest by pharmaceutical industries in new antimicrobial investment, as they view research for new antimicrobials as “low profit” and believe that resistance will develop for new antimicrobials sooner or later. As a result, they prefer to invest in the development of other drugs for chronic diseases in addition to those used to improve lifestyles. As antimicrobial resistance increases in many parts of the world, it becomes increasingly important to not just search for new antimicrobial agents but to come up with strategies to further reduce the emergence and rate of resistance or possibly counter the speedy development of antibiotic resistance by pathogenic microorganisms. It is mentioned that unsuccessful treatment of bacterial infections associated with antibiotic resistance claims at least 700,000 every year globally, and is projected to be associated with 10 million deaths per year by 2050. Given this very serious projection, it becomes indisputably critical for innovations in antibiotic drug technology and the valuation of new treatments to curb the antibiotic resistance menace.This is critical because failure to address antibiotic resistance jeopardizes the achievement of several Sustainable Development Goals (SDGs) (such as poverty reduction, inequality reduction, clean water, and sanitation) and undermines previous progress. In this review, immuneantibiotics, the inhibition of SOS response, and hydrogen sulfide as biochemical underlying networks leading to universal antibiotic resistance were explored and discussed as positive strategies.
Biochemical Approaches to Reducing Resistance and Increasing Antibiotic Susceptibility
Recent research has turned towards innovative strategies to combat antibiotic-resistant bacteria without necessitating the development of new antibiotics. This shift is particularly relevant given the significant costs and challenges associated with discovering and bringing new antibiotics to market. The primary goal of this approach is to neutralize the inherent resistance mechanisms of microorganisms, thereby enhancing the efficacy of existing antibiotics. This strategy not only promises to make previously approved antibiotics more effective but also offers a potential pathway to deliver better treatment options for infectious diseases at a reduced cost.
Understanding the mechanisms by which antibiotics act, along with the various bacterial resistance mechanisms, is essential for developing these alternative therapies. Each class of bactericidal antibiotic operates through unique mechanisms that target specific components of bacterial cells, ultimately leading to cell death. Different antibiotics influence bacterial physiology in various ways, such as by disrupting membrane integrity or inhibiting crucial cellular pathways. However, the intricate molecular networks that bacteria utilize to respond to antibiotic exposure and facilitate their survival remain less understood.
The process of antibiotic-mediated bacterial killing initiates with the drug’s interaction with its target within the bacterial cell, which triggers a series of biochemical and structural changes. As drug-resistant bacteria continue to evolve and proliferate, it becomes increasingly important to grasp how existing antibiotics induce bacterial death to identify new therapeutic avenues. Research indicates that all antibiotics, regardless of their primary targets, induce a secondary effect: the production of reactive oxygen species (ROS). These free radicals can inflict significant damage on bacterial DNA and proteins if not effectively neutralized.
Studies by Kohanski et al. have shown that bactericidal antibiotics across various classes lead to the formation of ROS when tested against both Gram-positive and Gram-negative bacteria. This phenomenon is not observed with bacteriostatic antibiotics, which do not generate hydroxyl radicals. Further investigations have clarified that the formation of hydroxyl radicals arises from a Fenton-like reaction involving ferrous iron and peroxide, resulting in double-strand DNA breaks—one of the principal mechanisms of peroxide-induced bacterial death.
Additionally, research suggests that the SOS response—a DNA repair mechanism activated by oxidative stress—plays a crucial role in bacterial resistance. This response can lead to the development of persister cells, which exhibit tolerance to antibiotics. Studies indicate that antibiotic exposure can trigger the SOS response, with mutants unable to form iron-sulfur clusters showing reduced susceptibility to bactericidal agents. The proteins involved in this repair process, such as RecA and LexA, facilitate the repair of damaged DNA, which can inadvertently promote genetic mutations contributing to antibiotic resistance.
The connection between ROS production and the SOS response underscores the potential for developing new therapeutic strategies that target these mechanisms. Inhibitors of the SOS response could prevent the development of antibiotic resistance, especially when used alongside sub-lethal concentrations of antibiotics. Current research includes the exploration of RecA inhibitors, which may enhance the effectiveness of bactericidal antibiotics while also mitigating resistance development.
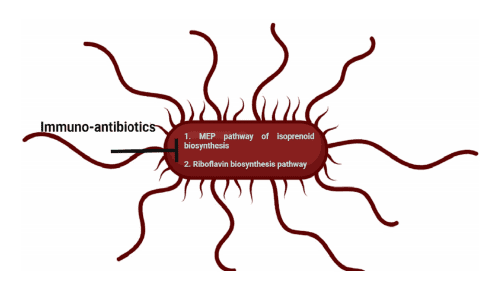
Figure 1. Immuno-antibiotics inhibit the non-mevalonate or methyl-D-erythritol phosphate (MEP) pathway of isoprenoid biosynthesis and riboflavin biosynthesis pathway in bacteria.
The Role of Hydrogen Sulfide (H₂S) in Antibiotic Resistance
Another critical area of research involves the role of endogenous hydrogen sulfide (H₂S) production in conferring antibiotic resistance across various bacterial species. Several studies have demonstrated that H₂S synthesis provides a protective effect against diverse antibiotics, contributing to the tolerance or resistance observed in many clinical isolates, including Staphylococcus aureus and Escherichia coli.
H₂S is believed to inhibit ROS generation by interfering with the Fenton reaction and enhancing the activity of ROS-scavenging enzymes. Genetic or pharmacological disruption of H₂S biosynthesis pathways has been shown to increase antibiotic sensitivity, indicating that targeting the H₂S pathway could enhance antibiotic effectiveness and reduce resistance.
The Fenton reaction, which leads to double-strand DNA breaks, is recognized as a primary mechanism for bacterial death resulting from antibiotic-induced oxidative stress. H₂S appears to bolster the activities of antioxidant enzymes such as catalase and superoxide dismutase, thereby enhancing bacterial resilience against oxidative damage. Research has confirmed that H₂S is synthesized in key pathogens through various enzymes, and this endogenous production facilitates a dual protective mechanism by both inhibiting oxidant production and activating antioxidant defenses.
However, it is important to note that exogenous H₂S does not always confer the same protective benefits as endogenous H₂S. Studies indicate that exogenous H₂S can be cytotoxic to certain bacteria, including Acinetobacter baumannii, which lacks the genes necessary for H₂S synthesis. When antibiotic-sensitive A. baumannii is treated with H₂S-releasing compounds alongside antibiotics, the bactericidal effect is significantly enhanced compared to the use of antibiotics alone.
Recent developments in H₂S-releasing compounds suggest their potential application in clinical settings to improve antibiotic efficacy and combat resistance in bacteria that do not produce H₂S. The safety profiles of these compounds are currently being evaluated, highlighting their promise as adjunct therapies to enhance the effectiveness of existing antibiotics.
Conclusion
The challenges posed by antibiotic resistance necessitate innovative approaches that leverage our understanding of bacterial mechanisms and antibiotic action. By focusing on biochemical pathways such as the SOS response and the role of hydrogen sulfide in antibiotic resistance, researchers are developing strategies that could significantly enhance the effectiveness of current antibiotics. These methods not only hold the potential to revitalize existing treatments but also pave the way for new therapeutic avenues to combat the growing threat of antibiotic-resistant infections. Through these advancements, we may achieve better clinical outcomes and improved patient care in the face of increasingly resistant microbial pathogens.
Reference
- Nwobodo, D. C., Ugwu, M. C., Anie, C. O., Al-Ouqaili, M. T. S., Ikem, J. C., Chigozie, U. V., & Saki, M. (2021). Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. Journal of Global Antimicrobial Resistance, 29, 1-10.
- Ng, S. Y., Ong, K. X., Surendran, S. T., & et al. (2020). Hydrogen sulfide sensitizes Acinetobacter baumannii to killing by antibiotics. Frontiers in Microbiology, 11, 1875.
- Mendes, S. S., Miranda, V., & Saraiva, L. M. (2021). Hydrogen sulfide and carbon monoxide tolerance in bacteria. Antioxidants, 10(5), 729.
- Zaorska, E., Tomasova, L., Koszelewski, D., Ostaszewski, R., & Ufnal, M. (2020). Hydrogen sulfide in pharmacotherapy, beyond the hydrogen sulfide-donors. Biomolecules, 10(2), 323.
- Miller, L. S., Fowler, V. G., Shukla, S. K., Rose, W. E., & Proctor, R. A. (2020). Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics, and bacterial evasion mechanisms. FEMS Microbiology Reviews, 44(1), 123-153.
- Wallace, J. L., Nagy, P., Feener, T. D., & et al. (2020). A proof-of-concept, phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide-releasing anti-inflammatory drug. British Journal of Pharmacology, 177, 769-777.
- Zhu, Z., Chambers, S., Zeng, Y., & Bhatia, M. (2022). Gases in sepsis: Novel mediators and therapeutic targets. International Journal of Molecular Sciences, 23(7), 3669.