Combatting the Global Crisis of Antibiotic Resistance: Strategies for a Healthier Future
Abstract
Antibiotic resistance has become one of the greatest threats to global public health, making once-curable infections increasingly difficult to control. Overuse and misuse of antibiotics, particularly in healthcare and agriculture, and poor infection control practices have accelerated the emergence of resistant strains. Key pathogens such as ESKAPE organisms are at the heart of a growing crisis, posing a serious risk to vulnerable populations. This article explores the key drivers of antibiotic resistance, the most critical pathogens involved, and the far-reaching implications for public health. It also discusses potential solutions to this growing problem, including improved antibiotic stewardship, investments in the development of new antibiotics, alternative therapies, and strengthened infection control measures. Global collaboration and a multi-pronged approach are essential to maintain the effectiveness of antibiotics and safeguard modern medicine.
Introduction to Antibiotic Resistance
Antibiotic resistance is one of the most pressing public health issues of our time. It occurs when bacteria evolve mechanisms to resist the drugs that once killed them or inhibited their growth. This phenomenon is fueled by the overuse and misuse of antibiotics, leading to a dramatic increase in the number of resistant bacterial strains. As a result, infections that were once easily treatable are becoming more difficult to manage, leading to longer hospital stays, increased medical costs, and higher mortality rates.
The discovery of antibiotics in the early 20th century revolutionized medicine, transforming the treatment of bacterial infections. Diseases such as pneumonia, meningitis, and tuberculosis, which were once fatal, became manageable with the advent of antibiotics. However, the excessive and inappropriate use of these drugs has accelerated the development of resistance, making previously treatable infections life-threatening once again.
Antibiotic resistance is not a new issue, but it has intensified in recent decades. The primary causes include the over prescription of antibiotics, especially in cases where they are not needed, such as viral infections, and the failure to complete prescribed antibiotic courses. Additionally, the use of antibiotics in agriculture and animal farming—where they are often administered to healthy animals to promote growth—has contributed to the rise of resistant bacteria in the environment.
The spread of antibiotic resistance poses a serious threat to global health security. Infections caused by resistant bacteria are more difficult to treat, and the lack of new antibiotics makes it challenging to manage emerging resistant strains. Without urgent action, antibiotic resistance could lead to a post-antibiotic era where even minor infections could be fatal. Addressing this issue requires a multifaceted approach, including better stewardship of existing antibiotics, increased investment in research and development, and global cooperation to limit the spread of resistant pathogens.
Key Factors Driving Antibiotic Resistance
Antibiotic resistance is primarily driven by human actions that facilitate the survival and spread of resistant bacteria. The most significant factors contributing to this global health threat include the overuse and misuse of antibiotics in both healthcare and agriculture, as well as poor infection control practices in hospitals.
In healthcare settings, antibiotics are often prescribed unnecessarily or inappropriately. This occurs when antibiotics are prescribed for viral infections such as the common cold or influenza, for which antibiotics are ineffective. Additionally, patients may not complete their prescribed antibiotic course, leading to incomplete eradication of the bacteria. This incomplete treatment allows surviving bacteria to adapt and develop resistance. Studies show that nearly half of all antibiotic prescriptions are unnecessary, particularly in outpatient settings, further contributing to resistance.
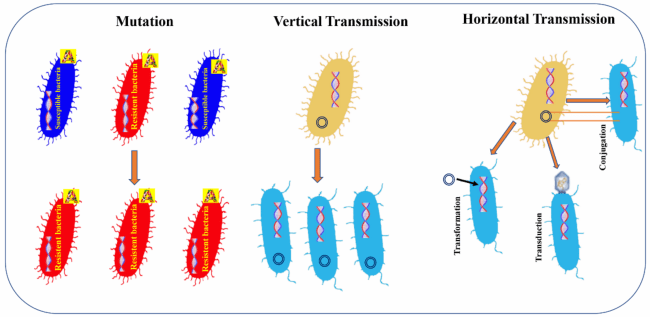
Figure 1. How antibiotic resistance spread.
Another major factor in the rise of antibiotic resistance is the use of antibiotics in agriculture. In many parts of the world, antibiotics are used not only to treat infections in livestock but also to promote growth in healthy animals. This practice contributes to the development of resistant bacteria, which can then spread to humans through the food supply. Resistant pathogens from farm animals can contaminate meat, water, and vegetables, thus entering the human food chain. Furthermore, the use of antibiotics in farming increases the chances of resistance in environmental bacteria, which can be transmitted to humans through contact with contaminated soil or water.
Poor infection control practices, particularly in healthcare environments, also contribute to the spread of resistant bacteria. Inadequate hand hygiene, improper sterilization of medical equipment, and overcrowding in hospitals can all facilitate the transmission of resistant strains between patients. This is particularly problematic in hospitals and nursing homes where vulnerable patients, such as the elderly and immunocompromised individuals, are at higher risk of acquiring infections.
To combat antibiotic resistance, it is crucial to address these factors by improving antibiotic stewardship, reducing the overuse of antibiotics in agriculture, and enhancing infection control measures in healthcare settings.
Most Critical Pathogens in Antibiotic Resistance
Antibiotic resistance has created a significant challenge in managing bacterial infections, particularly from a subset of highly resistant pathogens known as the ESKAPE organisms. These pathogens are among the most critical and pose a major threat to public health worldwide due to their ability to evade the effects of commonly used antibiotics. The ESKAPE group includes Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. These pathogens are responsible for a wide range of infections, including bloodstream infections, pneumonia, urinary tract infections, and surgical site infections.
One of the most concerning pathogens is Methicillin-resistant Staphylococcus aureus (MRSA), which is resistant to most penicillins and other antibiotics. MRSA is particularly problematic in healthcare settings, where it can cause severe infections in patients with weakened immune systems. It can also spread easily through direct contact or contaminated surfaces. Klebsiella pneumoniae is another major concern, particularly as it has developed resistance to carbapenems, a class of antibiotics considered last-resort drugs for treating multidrug-resistant infections. K. pneumoniae is associated with hospital-acquired infections, including ventilator-associated pneumonia and bloodstream infections, and can rapidly spread in healthcare environments.
Acinetobacter baumannii and Pseudomonas aeruginosa are notorious for their ability to survive in harsh hospital environments, including on medical equipment and surfaces. These pathogens are often resistant to multiple antibiotics, making infections caused by them difficult to treat. In addition, P. aeruginosa is frequently responsible for chronic infections in patients with cystic fibrosis or burn wounds.
The rapid emergence of resistance in these pathogens underscores the urgency of developing new antibiotics and alternative treatments. In the absence of novel antimicrobial agents, the medical community is increasingly turning to combination therapies, increased surveillance, and improved infection control strategies to combat the spread of these dangerous bacteria.
The Impact of Antibiotic Resistance on Public Health
The growing problem of antibiotic resistance has profound implications for global public health, affecting the treatment and management of infectious diseases. As bacteria evolve mechanisms to resist the effects of commonly used antibiotics, infections that were once easily treatable are becoming harder to manage, leading to longer hospital stays, increased medical costs, and higher mortality rates. The impact is felt not only in developed countries but also in low- and middle-income nations, where the burden of resistant infections is particularly severe due to limited healthcare resources and access to newer treatments.
One of the most significant consequences of antibiotic resistance is the increased duration and complexity of treatment. Infections caused by resistant bacteria often require more aggressive and prolonged therapies, which can lead to complications such as side effects from stronger drugs or the need for hospitalization. For example, resistant strains of Escherichia coli or Klebsiella pneumoniae can cause urinary tract infections, pneumonia, and bloodstream infections that are difficult to treat with standard antibiotics. In these cases, doctors may need to rely on second- or third-line antibiotics, which are often less effective and more toxic, and may require intravenous administration.
The economic burden of antibiotic resistance is also substantial. Infections caused by resistant bacteria often result in longer hospitalizations, the need for more expensive medications, and increased healthcare utilization. The World Bank has estimated that by 2050, antibiotic resistance could push 28 million people into extreme poverty due to increased medical costs and lost productivity. Furthermore, resistant infections can delay or complicate surgeries, cancer treatments, and organ transplants, procedures that often rely on antibiotics to prevent or treat infections.
Beyond the direct impact on individual patients, antibiotic resistance poses a threat to the effectiveness of modern medicine itself. The risk of untreatable infections undermines advances in surgery, cancer care, and chronic disease management, ultimately endangering the safety of medical procedures that rely on effective infection control. Therefore, combating antibiotic resistance is not only a matter of addressing individual infections but of preserving the progress made in modern medicine.
Solutions and the Road Ahead
Antibiotic resistance is the ability of bacteria to resist exposure to antibiotics designed to kill them or inhibit their growth. Although antibiotic resistance is a natural process due to genetic changes in the bacteria following antibiotics exposure, however, this phenomenon is being accelerated through the overuse and misuse of antibiotics.Addressing the escalating threat of antibiotic resistance requires a comprehensive and multi-pronged approach. Governments, healthcare organizations, researchers, and the general public must all play a role in mitigating the impact of this global crisis. Efforts must focus on improving antibiotic stewardship, enhancing research into new antibiotics, and promoting better infection control practices. Furthermore, international collaboration is critical to ensure effective solutions and prevent the spread of resistant bacteria.
Overuse of antibiotics causes susceptible bacteria to be killed and allows drug-resistant bacteria to proliferate. Poor sanitation, poor infection control and the use of antibiotics in farm animals are among
the main reasons for the spread of antimicrobial resistance. In addition, there are novel and often underrecognized mechanisms of resistance that further contribute to drug resistance such as the heteroresistance (HR) and the mutant prevention concentration (MPC). The first of these two factors can be defined as resistance to certain antibiotics by a preexisting subpopulation of resistant cells, within a larger population of antimicrobial-susceptible microorganisms. This sub-population of resistant cells can rapidly replicate in the
presence of a given antibiotic whereas the susceptible microorganisms are killed. Recent reports indicate that heteroresistance is very common for several bacterial species and
classes of antibiotics. The second one, known as MPC, represents a threshold above which the selective proliferation of resistant mutants is expected to occur only rarely. Traditionally, the minimum inhibitory concentration (MIC) has been widely used to determine the susceptibility and resistance of bacteria to antimicrobials. However, MIC represents one parameter of resistance, but not all. Due to spontaneous mutations, even after exposure of cells to an antibiotic at MIC levels, a subpopulation of antibiotic-resistant mutants often remains. Increasing the concentration of the antibiotic above the MIC will result in a value that will kill all mutants. This concentration is the MPC that can be defined as the MIC of the least-susceptible, single-step mutant. In this context, it is essential to determine the MPC/MIC ratio in order to prevent the emergence of mutant. The ESKAPE pathogens represent deadly bacteria with rapidly growing multi-drug
resistant properties. Although these bacteria are genetically different, the resistance strategies that underlie the emergence and persistence of these pathogens are widely shared among them including decreased drug uptake, drug target alteration, drug inactivation and drug efflux pumps activation. To limit the spread of ESKAPE pathogens and antibiotic resistance more generally, it has become imperative to be more careful in surveillance and implementation of antimicrobial stewardship in both human health and food animals.
Antibiotic stewardship refers to the careful management of antibiotic use to reduce unnecessary prescriptions and ensure that antibiotics are only used when appropriate. Healthcare providers must be educated about the risks of over-prescribing antibiotics, particularly for viral infections where they are ineffective. Additionally, public awareness campaigns can help patients understand the importance of following prescribed antibiotic regimens and avoiding self-medication. Improving diagnostic tools to better distinguish between bacterial and viral infections would also aid in reducing inappropriate antibiotic prescriptions.
One of the most promising strategies to combat antibiotic resistance is the development of new antibiotics and alternative therapies. The rapid emergence of resistant strains has outpaced the development of new antimicrobial agents, leaving a significant gap in available treatment options. Encouraging pharmaceutical companies to invest in the research and development of novel antibiotics is crucial. Governments and international organizations can play a role by providing incentives such as subsidies, tax breaks, or patent protections for companies working on new antibiotics. Moreover, exploring alternative treatments such as bacteriophage therapy or antimicrobial peptides could offer new avenues for combating infections.
Infection control practices must be strengthened across healthcare settings, particularly in hospitals and nursing homes where vulnerable populations are at greatest risk. This includes improving hygiene practices, such as handwashing, and ensuring proper sterilization of medical equipment. Enhanced surveillance systems can also help track the spread of resistant pathogens and identify outbreaks early.
In conclusion, tackling antibiotic resistance requires global cooperation, improved antibiotic stewardship, investment in new treatments, and enhanced infection control measures. Without these efforts, we risk returning to an era where even minor infections could be deadly.
References
- Mancuso, G., Midiri, A., Gerace, E., & Biondo, C. (2021). Bacterial antibiotic resistance: The most critical pathogens. Pathogens, 10(10), 1310.
- Boucher, H. W., et al. (2009). Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clinical Infectious Diseases, 48(1), 1–12.
- Davies, J., & Davies, D. (2010). Origins and evolution of antibiotic resistance. Microbiology and Molecular Biology Reviews, 74(3), 417–433.
- Laxminarayan, R., et al. (2013). Antibiotic resistance—the need for global solutions. The Lancet Infectious Diseases, 13(12), 1057–1098.
- O’Neill, J. (2016). Tackling drug-resistant infections globally: Final report and recommendations. Review on Antimicrobial Resistance.
- Ventola, C. L. (2015). The antibiotic resistance crisis: Part 1: Causes and threats. Pharmacy and Therapeutics, 40(4), 277–283.
- Magill, S. S., Edwards, J. R., Beldavs, Z. G., Dumyati, G., Jr., Kainer, M. A., Jones, R. N., … Fridkin, S. K. (2014). Multistate point-prevalence survey of health care-associated infections. New England Journal of Medicine, 370(13), 1198–1208.