Current Research of HIF Inhibitors
Abstract
Hypoxia-inducible factors (HIFs) are transcription factors that play a crucial role in oxygen homeostasis. They regulate the expression of genes involved in various cellular processes such as metabolism, angiogenesis, and erythropoiesis. Under normal oxygen levels, HIFs are degraded rapidly through a ubiquitin-proteasome system[1]. However, when oxygen levels are low, HIFs stabilize and translocate to the nucleus, where they bind to hypoxia response elements (HREs) in the promoter regions of target genes and initiate their transcription.
HIFs have been implicated in the pathogenesis of various diseases, including cancer, cardiovascular diseases, and neurodegenerative disorders. In cancer, HIFs promote tumor growth and metastasis by promoting angiogenesis and altering cellular metabolism to meet the energy demands of rapidly proliferating cells[2]. HIFs are also involved in the development of ischemic diseases such as stroke and myocardial infarction, where they promote tissue damage by inducing inflammation and oxidative stress. In neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease, HIFs have been shown to play a role in neuronal damage and apoptosis.
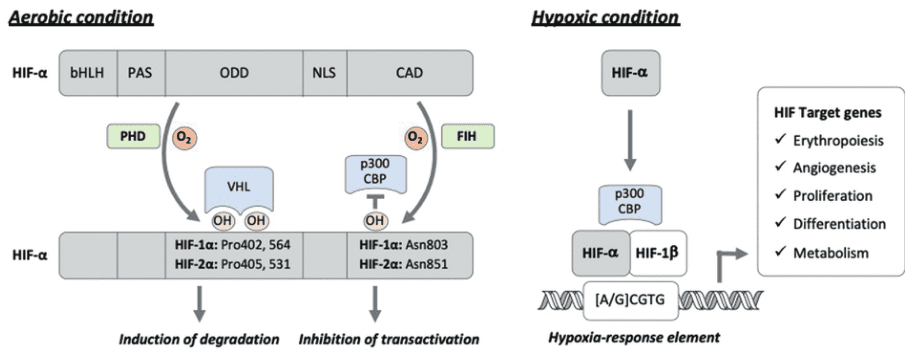
Figure 1 The HIF pathway under normoxic and hypoxic conditions[3]
Given their role in disease pathogenesis, HIFs have emerged as potential therapeutic targets. Small molecule inhibitors of HIFs have been developed and tested in preclinical models, with promising results in cancer and ischemic diseases. However, the development of HIF inhibitors as therapeutics is challenging, given the complex and context-dependent role of HIFs in cellular processes.
Overview of the potential therapeutic applications of HIF inhibitors
Hypoxia-inducible factors (HIFs) have emerged as potential therapeutic targets in various diseases, including cancer, ischemic diseases, and inflammatory disorders. HIF inhibitors are small molecules that target the HIF pathway and have the potential to modulate the expression of genes involved in oxygen homeostasis and other cellular processes.
In cancer, HIF inhibitors have been shown to block the formation of new blood vessels, a process known as angiogenesis, which is essential for tumor growth and metastasis. HIF inhibitors can also modulate cellular metabolism to inhibit the growth and proliferation of cancer cells. Preclinical studies have shown promising results for HIF inhibitors in a variety of cancer types, including renal cell carcinoma, breast cancer, and glioblastoma.
In ischemic diseases such as stroke and myocardial infarction, HIF inhibitors have the potential to reduce tissue damage by inhibiting the inflammatory response and promoting tissue repair. HIF inhibitors can also stimulate the formation of new blood vessels to improve tissue perfusion in ischemic conditions.
In conditions characterized by inflammation, such as rheumatoid arthritis and inflammatory bowel disease, HIF inhibitors have demonstrated the ability to decrease the production of inflammatory cytokines and regulate the immune response. Additionally, HIF inhibitors can enhance tissue oxygenation and facilitate tissue repair in the context of inflammatory conditions.
HIF-1α inhibitors for cancer treatment
Several research institutions and universities have made significant advances in identifying and developing inhibitors for hypoxia-inducible factor-1 alpha (HIF-1α) as a therapeutic target for angiogenesis-related diseases such as cancer, diabetic retinopathy, and rheumatoid arthritis.
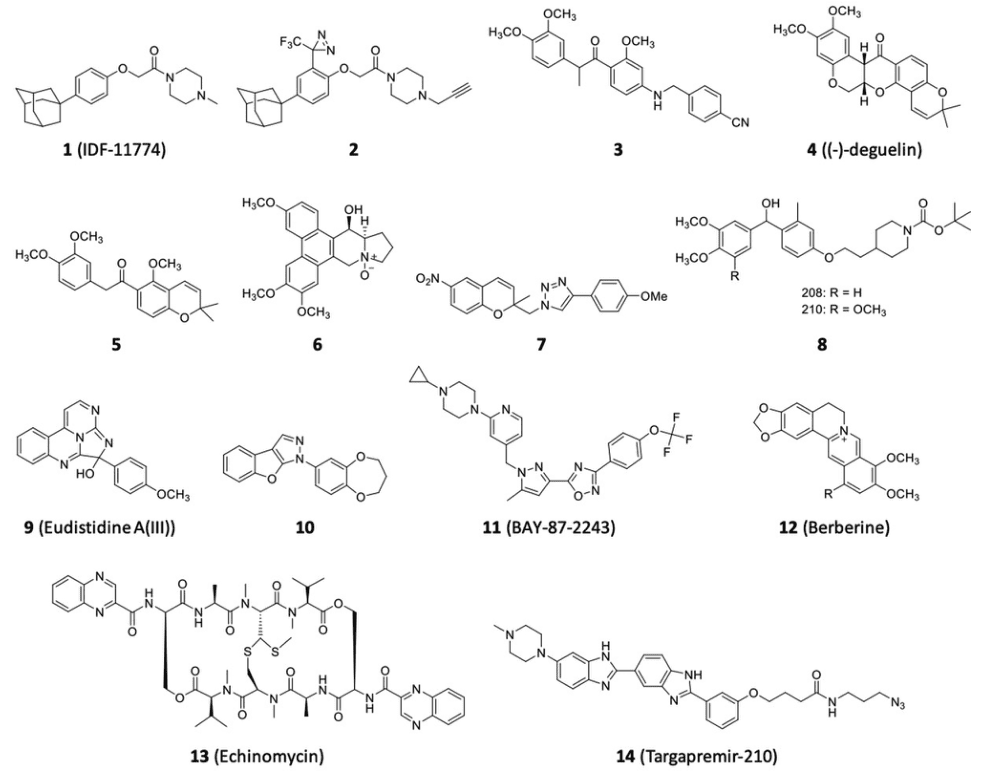
Figure 2 HIF-1α inhibitors for the treatment of cancer[3]
Researchers from Dongguk University and the Korea Research Institute of Bioscience and Biotechnology reported the identification of HSP70 as a target protein of IDF-11774, a novel HIF-1 inhibitor. IDF-11774 reduced HIF-1α accumulation and inhibited HIF-1α-mediated target gene expression[3]. Chemical probe 2 was developed to clarify the mechanism of action of IDF-11774, and it was found that the inhibitor binds to the allosteric site of HSP70, suppressing its chaperone activity, which reduces refolding of HIF-1. Compound 3 was disclosed by the Seoul National University R&DB Foundation as a HIF-1α inhibitor[4]. It showed enhanced inhibitory activity compared to compound 5, which was developed based on the structure of (-)-deguelin[5]. Compound 3 effectively suppressed hypoxia-mediated retinal neovascularization.
The Research Institute of Fox Chase Cancer Center disclosed drug combinations comprising a cyclin-dependent kinase inhibitor (CDKI) and HSP90 inhibitors for treating cancer. CDKI stabilizes HIF-1α through direct phosphorylation of its Ser668 residue in a VHL-independent manner, and HSP90 is also known to be a VHL-independent HIF-1α stabilizer that has been recognized as a therapeutic target in cancer[6,7]. The current patent application suggests that crosstalk between CDK1-mediated and HSP90-mediated HIF-1α stabilization could be a therapeutic target.
Macau University of Science and Technology disclosed a method of isolating phenanthroindolizidine alkaloids from Tylophora atrofolliculata that displayed HIF-1 inhibitory activity. Among the 11 new phenanthroindolizidine alkaloids isolated, compound 6 showed exceptional HIF-1 inhibitory activity, which is comparable to that of Manassantin B.
Kyungpook National University has patented a series of 1,2,3-triazole derivatives as HIF-1α inhibitors for the treatment of angiogenesis-related diseases. Compound 7 showed the most potent inhibitory activity against hypoxia-induced HIF-1α accumulation in HEK-293 cells and A549 cells. Compound 7 enhanced hydroxylation and ubiquitination of HIF-1α, resulting in the reduction of HIF-1α levels[8]. Compound 7 suppressed the expression of the HIF-1α target gene VEGF and inhibited VEGF-induced angiogenesis in human umbilical vein endothelial cells. The combination of compound 7 and the EGFR inhibitor gefitinib showed a synergistic inhibitory effect on tumor growth through suppression of angiogenesis in a Lewis lung carcinoma allograft mouse model.
Georgia State University Research Foundation and Emory University have disclosed benzhydrol derivatives for the treatment of cancers related to HIFs. The researchers found that compound 8, with an IC50 of 0.5 nM, significantly inhibits the activity of mitochondrial complex I, which interferes with the normal function of the electron transport chain, leading to cell death through the generation of reactive oxygen species.
The National Institute of Health has also identified small molecules that inhibit HIF-1 activity. Compound 9, which was isolated from a marine ascidian Eudistoma sp., was found to inhibit HIF-1α subunit and transcriptional co-activator protein p300 interaction, with an IC50 of 75 μM. The scaffold of histidine A, the compound from which compound 9 was synthesized, may have potential as a therapeutic lead compound or chemical probe for studying p300/HIF-1α interactions under hypoxia.
Researchers from the Tokyo Institute of Technology have developed benzofuropyrazoles that inhibit the transcriptional activity of HIF-1α under hypoxia. For example, compound 10 was found to inhibit the transcriptional activity of HIF-1α with an IC50 of 0.24 µM, without suppressing HIF-1α accumulation under hypoxia. This suggests that compound 10 acts on the transcriptional process in the nucleus.
Xiamen University researchers have developed medicaments comprising a combination of ROS-inducing agents and HIF-1 inhibitors for the treatment of cancer. BAY-87-2243, a HIF-1 inhibitor developed by Bayer Pharma AG, was found to have antiproliferative activity and to induce apoptosis in human malignant melanoma A375 cells, as well as to reduce metastasis and tumor growth in C57BL/6 J nude mice bearing A375 cancer xenografts with no effect on colon length, body, and spleen weight.
Berberine, a benzylisoquinoline alkaloid, has been found to have a wide variety of biological activities and is currently being investigated for its potential to treat several diseases. Macau University of Science and Technology researchers have developed berberine derivatives that display stronger cytotoxicity toward MCF-7 breast cancer cells and an increased inhibitory effect on hypoxia-induced HIF-1 transcriptional activity compared to berberine itself.
The potential of HIF inhibitors in the treatment of various diseases, including cancer, has been explored by the Children’s Research Institute at the Children’s National Medical Center. Echinomycin, a quinoxaline antibiotic, has been investigated as a HIF inhibitor for the treatment of graft versus host disease, proliferative disease, leukemia, cancer, and autoimmune disease. Echinomycin directly inhibits the binding of HIF-1α to the cis-element HRE, leading to reduced expression of HIF-1 target genes. A liposomal drug formulation of echinomycin has been disclosed for the treatment of GvHD and other diseases.
Ongoing research aims to identify novel HIF-1 inhibitors, as the dysregulation of this transcription factor has been linked to numerous diseases. Several promising candidates have been identified, targeting different aspects of the HIF-1 pathway and showing potential in preclinical studies. However, further research is necessary to evaluate their safety and efficacy in human clinical trials. The development of effective HIF-1 inhibitors has the potential to revolutionize cancer therapy and improve the quality of life for millions of people worldwide.
HIF-2α inhibitors for the treatment of cancer
Pelton Therapeutics has made public several patent applications that describe inhibitors of HIF-2α and their application in cancer treatment. The company claims a range of aromatic compounds as HIF-2α inhibitors, including compound 20 which displays potent inhibitory activity against HIF-2α. In the 786-O xenograft model, compound 20 selectively inhibited HIF-2α activity, resulting in a reduction in tumor growth of up to 91%. In addition, Pelton Therapeutics has reported a series of 6,7-dihydro-5 H-cyclopenta[c]pyridin-7-ol compounds as inhibitors of HIF-2α, of which compounds 21, 22, and 23 displayed potent HIF-2α inhibitory activity[9]. These compounds significantly suppressed the mRNA levels of HIF-2α target genes such as VEGF, CCND1, and PAI1. Another patent application from Pelton Therapeutics described tricyclic inhibitors of HIF-2α for the treatment of HIF-2α-related diseases. Compound 24 was found to have potent inhibitory activity against HIF-2α transcriptional activation and strongly reduced VEGF production in 786- O cells.
A further patent application from Pelton Therapeutics suggested using HIF-2α inhibitors in combination with immunotherapeutic agents for cancer treatment. In mouse melanoma and colon carcinoma xenograft models, PT2385 displayed a synergistic inhibitory effect on tumor growth in combination with anti-PD-1 and anti-cytotoxic T lymphocyte-associated protein (CTLA)-4 antibodies, respectively. The same application also confirmed that PT2385 disrupted the binding of HIF-2α and HIF-1β.
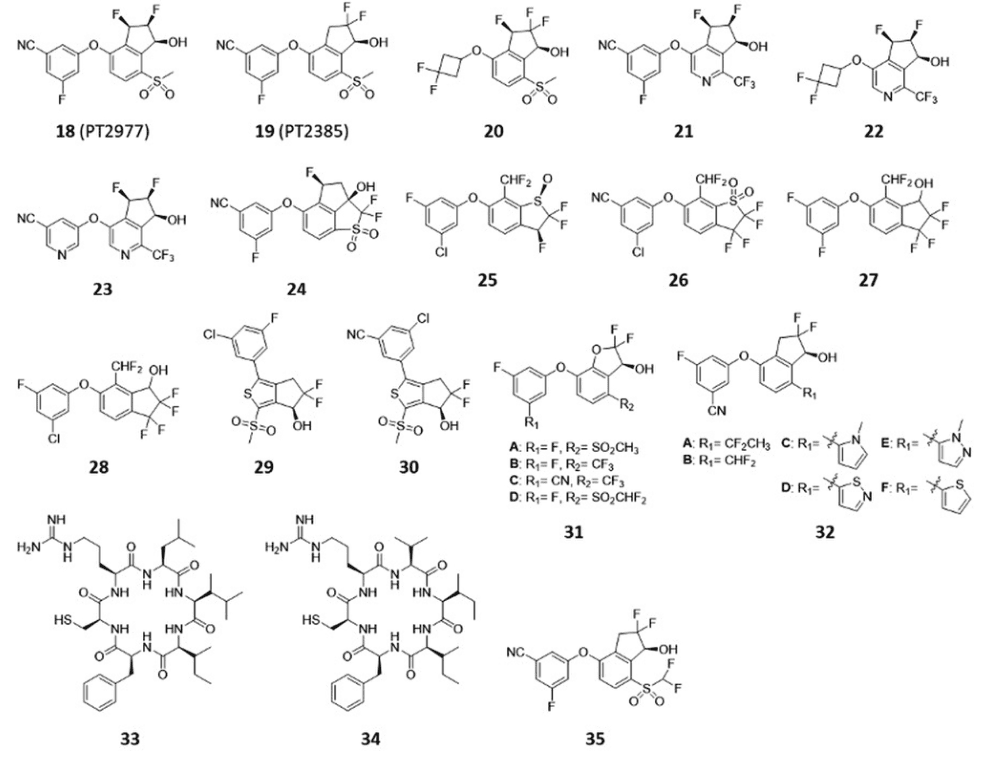
Figure 3 HIF-2α inhibitors for the treatment of cancer[3]
Pelton Therapeutics has also disclosed information about the solid dispersions and pharmaceutical compositions of PT2977 in a separate patent application. A complete dissolution of PT2977 was observed in 15 minutes for both 10 and 40 mg tablets in a common blend of tablet formulations. Nikang Therapeutics claimed the use of dihydrobenzo[b]thiophene compounds and a series of indane analogs as HIF-2α inhibitors for cancer treatment in separate patent applications. Among the claimed compounds, compounds 25 and 26, and compounds 27 and 28, respectively, showed potent inhibitory effects on VEGF expression. However, the mechanisms of action of these claimed inhibitors of HIF-2α were not discussed in the patent applications.
Finally, a series of thiophene derivatives have been reported by Merck Patent GmbH and Selvita S.A. as HIF-2α inhibitors for the treatment of cancer. Compounds 29 and 30 displayed potent inhibitory activity against HIF-2α in a 786-O cell-based HRE reporter gene assay. Moreover, these compounds inhibited the interaction of PAS-B domains of HIF-2α and HIF-1β. Frontera US Pharmaceuticals reported the preparation of 2,3-indene and benzofuran compounds as HIF-2α inhibitors for cancer treatment. These compounds were found to affect HIF-2α activity in a hypoxia-response element (HRE) luciferase assay.
Pelton Therapeutics has filed a patent application for the use of a HIF-2α inhibitor in the treatment of glioblastoma. One of the compounds claimed, compound 35, demonstrated potent inhibitory activity against HIF-2α in various assays and reduced tumor growth in a GBM tumor xenograft model. Administration of compound 35 at 100 mg/kg b.i.d. also increased the survival rate of the tumor-bearing mice.
Moreover, a patent application from the University of Texas claimed the identification of potential biomarkers of response to HIF-2α inhibition for the prediction and treatment of cancer. The authors of the study analyzed gene expression changes in patient tumors that were either resistant or sensitive to the HIF-2α inhibitor compound 35. In patient-derived xenograft models, they found that compound 35-sensitive tumors exhibited higher levels of 11 genes (EPAS1, CPE, C1QL1, CXCR4, IGFBP1, INHBB, LOX, PTHLH, RDH13, SCL6A3, and SORCS3) and lower levels of 3 genes (HIF1A and HMGA1) compared to resistant tumors.
The identification of 11 potential biomarkers by the researchers could allow for personalized treatment strategies for cancer patients, predicting their response to HIF-2α inhibitor therapy. These biomarkers may also facilitate the development of new treatments targeting HIF-2α, leading to improved outcomes for patients with diverse cancer forms.
Summary
HIF, or hypoxia-inducible factor, is a critical regulator of oxygen homeostasis, and its dysregulation is associated with a range of human diseases, including ischemic cardiovascular disease, stroke, chronic lung disease, and cancer. Although inhibiting the HIF pathway holds promise for treating these diseases, the complexity of the HIF-1-related pathway has made it challenging to develop clinically available HIF inhibitors.
This review classifies HIF inhibitors into two types: those that inhibit the HIF-1α pathway and those that inhibit the HIF-2α pathway. Most HIF-1α inhibitors interact with HIF-1α indirectly, such as IDF-11774 (compound 1), (-)-deguelin and its derivatives (compounds 3–5), and compound 8, which interacts with the mitochondrial complex I to interfere with the normal functioning of the electron transport chain, resulting in cell death through the generation of reactive oxygen species. Compounds 10 and 12 also inhibit HIF-1α indirectly. In contrast, histidine A (III) (compound 9) and echinomycin (compound 13) directly interact with HIF-1α to inhibit its binding to p300 and cis-element HRE, respectively. Direct inhibitors are considered less complex to investigate the action mechanism required for clinical application.
Regarding the compounds that inhibit the HIF-2α pathway, most suppress HIF-2α activity rather than protein levels by directly disrupting the binding of HIF-2α and HIF-1β. For example, PT2385 (compound 19) binds to the allosteric site of HIF-2α to inhibit its activity, and compounds 29, 30, and 34 directly inhibit the interaction of PAS-B domains of HIF-2α and HIF-1β.
Recent studies have focused on the use of HIF inhibitors in the treatment of various diseases, including hypoxic pulmonary hypertension (HPH), circadian rhythm disorders, calcific aortic valve disease (CAVD), cerebrovascular accident (CVA), heterotopic ossification (HO), iron overload disorders, Crohn’s disease, ulcerative colitis, and thyroid eye disease. These applications are based on the regulation of oxygen by HIF signaling pathways.
Of particular interest is the finding that hypoxia promotes changes that contribute to an immunosuppressive phenotype defined by the presence of different types of immune cells within the microenvironment. B cells have been shown to contribute to cytotoxic T cell exhaustion and produce chemokines to attract more immunosuppressive regulatory T cells under hypoxia. Recent studies have shown that PT2385 (compound 19), a HIF-2α inhibitor, exerts a synergistic inhibitory effect on tumor growth when combined with an anti-PD-1 antibody, an immunotherapeutic agent, indicating that effective immunotherapy for solid tumors can counteract the immunosuppression caused by hypoxia in the tumor microenvironment.
Considering the effects of hypoxia on cancer cells, stromal cells, and effector immune cells is crucial for the development of successful treatments. Although inhibiting the HIF pathway is expected to be effective in treating many diseases, the complexity of this pathway has made it difficult to develop clinically available HIF inhibitors. Nevertheless, recent studies have identified several promising compounds that hold great potential for clinical use, particularly in combination with other therapies, such as immunotherapy, to improve patient outcomes.
Reference
- Ohh, M., Park, C. W., Ivan, M., Hoffman, M. A., Kim, T. Y., Huang, L. E., … & Kaelin, W. G. (2000). Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel–Lindau protein. Nature cell biology, 2(7), 423-427.
- Hewitson, K. S., McNeill, L. A., Riordan, M. V., Tian, Y. M., Bullock, A. N., Welford, R. W., … & Schofield, C. J. (2002). Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. Journal of Biological Chemistry, 277(29), 26351-26355.
- Ban, H. S., Uto, Y., & Nakamura, H. (2021). Hypoxia-inducible factor (HIF) inhibitors: a patent survey (2016–2020). Expert Opinion on Therapeutic Patents, 31(5), 387-397.
- Ban, H. S., Kim, B. K., Lee, H., Kim, H. M., Harmalkar, D., Nam, M., … & Won, M. (2017). The novel hypoxia-inducible factor-1α inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growth. Cell death & disease, 8(6), e2843-e2843.
- An, H., Lee, S., Lee, J. M., Jo, D. H., Kim, J., Jeong, Y. S., … & Suh, Y. G. (2018). Novel hypoxia-inducible factor 1α (HIF-1α) inhibitors for angiogenesis-related ocular diseases: Discovery of a novel scaffold via ring-truncation strategy. Journal of Medicinal Chemistry, 61(20), 9266-9286.
- Chang, D. J., An, H., Kim, K. S., Kim, H. H., Jung, J., Lee, J. M., … & Suh, Y. G. (2012). Design, synthesis, and biological evaluation of novel deguelin-based heat shock protein 90 (HSP90) inhibitors targeting proliferation and angiogenesis. Journal of medicinal chemistry, 55(24), 10863-10884.
- Warfel, N. A., Dolloff, N. G., Dicker, D. T., Malysz, J., & El-Deiry, W. S. (2013). CDK1 stabilizes HIF-1α via direct phosphorylation of Ser668 to promote tumor growth. Cell cycle, 12(23), 3689-3701.
- Calderwood, S. K., & Gong, J. (2016). Heat shock proteins promote cancer: it’s a protection racket. Trends in biochemical sciences, 41(4), 311-323.
- Park, K., Lee, H. E., Lee, S. H., Lee, D., Lee, T., & Lee, Y. M. (2017). Molecular and functional evaluation of a novel HIF inhibitor, benzopyranyl 1, 2, 3-triazole compound. Oncotarget, 8(5), 7801.
- Paul, W. E. H. N., & Yang, H. (2017). U.S. Patent No. 9,796,697. Washington, DC: U.S. Patent and Trademark Office.




