Abstract
Cyclin D1 is a key regulator of the cell cycle that plays a crucial role in the pathogenesis of various cancers. Traditionally known for its involvement in cell cycle progression, Cyclin D1 also influences tumor-stroma interactions and immune system modulation, contributing to tumor growth, metastasis, and immune evasion. Its overexpression and genetic amplification are commonly observed in several malignancies, including breast, head and neck, and liver cancers, and are associated with poor prognosis and therapeutic resistance. Recent studies have expanded the understanding of Cyclin D1’s function, highlighting its CDK-dependent and CDK-independent roles in modulating cell cycle regulators and transcription factors. Furthermore, Cyclin D1’s ability to affect the tumor microenvironment underscores its importance in promoting cancer progression. Given its central role in multiple oncogenic processes, Cyclin D1 has emerged as a promising therapeutic target. Targeting Cyclin D1 or its downstream signaling pathways, including CDK4/6 inhibition, represents a potential strategy to combat cancers characterized by its overexpression. Further research into Cyclin D1’s multifaceted roles may provide new avenues for personalized cancer treatment.
Introduction – The Central Role of Cyclin D1 in Cancer Pathogenesis
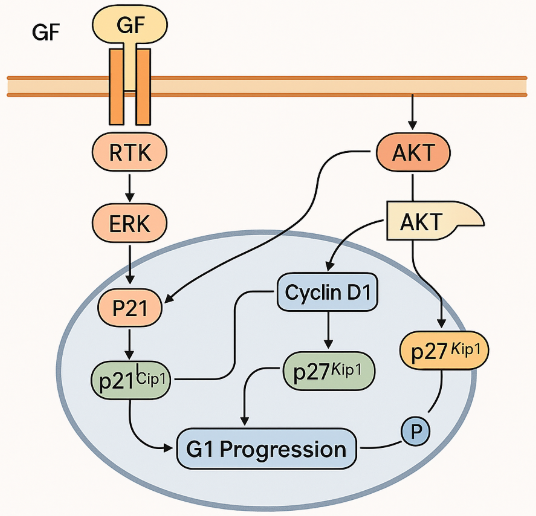
Cyclin D1, a key regulator of the cell cycle, plays a crucial role in the pathogenesis of various cancers, driving uncontrolled cell proliferation. It functions primarily in the G1 phase of the cell cycle, where it binds to cyclin-dependent kinases (CDKs), particularly CDK4 and CDK6, to phosphorylate the retinoblastoma (RB) protein. This phosphorylation releases the E2F transcription factors, promoting the transition from G1 to S phase and initiating DNA synthesis. In normal cells, Cyclin D1 expression is tightly controlled, ensuring proper cell cycle progression. However, in cancer cells, Cyclin D1 expression is often dysregulated, contributing to tumorigenesis.
Overexpression or amplification of the CCDN1 gene, which encodes Cyclin D1, is a common feature in several cancer types, including breast, lung, and head and neck cancers. This amplification leads to increased Cyclin D1 levels, resulting in hyperactivation of CDK4/6, disruption of the RB-E2F checkpoint, and the bypass of critical cell cycle controls. Consequently, cancer cells gain a growth advantage, proliferating uncontrollably. Cyclin D1 has been identified as a negative prognostic marker in many cancers, particularly in breast cancer, where its overexpression correlates with poor patient outcomes, including increased tumor recurrence and resistance to therapy.

Figure 2. It is the molecular diagram illustrating the mechanism of Cyclin D1 in cancer progression.
The role of Cyclin D1 in cancer initiation and progression appears complex and multifaceted, indicating that nowadays further investigations are needed for a more exhaustive comprehension of the therapeutic intervention targeting Cyclin D1-dependent mechanisms. Recent evidences strongly suggest the role of Cyclin D1 in the resistance to therapy and cancer progression. Cyclin D1 antagonizes BRCA1-mediated repression of estrogen receptor α (ERα)–dependent gene expression suggesting that silencing of CCND1 combined with PARP inhibitors may lead to substantial benefit for several type of cancer patients. In this concern, orthotopic ovarian cancer models respond better to olaparib treatment after knockdown of CCND1 gene compared with olaparib treatment alone.
Besides, Cyclin D1 and the autophagic degradation machinery are together linked to hepatocellular carcinoma (HCC) tumorigenesis. It is reported that rise of autophagy-dependent Cyclin D1degradation using amiodarone and rapamycin, represses tumor growth in both the orthotopic liver tumor and subcutaneous tumor xenograft models. The proliferative status of the induced tumors is not significantly different regardless of the Cyclin D1 localization in the nucleus or in the cytoplasm. Notably, an important role of cytoplasmic and membrane-associated Cyclin D1 for the regulation of cellular invasive capacity is emerged . Based on the first major studies, more recently authors demonstrated that Cyclin D1 plays a role in the dissemination of glioblastoma (GBM) in vivo. Using GBM xenografts, it is shown that invasive GBM cells, but not cells within the tumor masses, express a Cyclin D1 mostly located in the cytoplasm, and forced membrane accumulation of Cyclin D1 induces the number of invading cells. Cyclin D1’s role in cancer is not limited to cell cycle control. Recent studies have highlighted its involvement in tumor invasion and metastasis. Beyond the nucleus, Cyclin D1 is localized to the cytoplasm, where it interacts with the cell membrane, promoting cell adhesion, migration, and invasion—key steps in metastatic spread. Additionally, Cyclin D1 modulates the tumor microenvironment by influencing interactions between tumor cells and stromal components, further potentiating cancer progression. These multifaceted roles underscore Cyclin D1’s importance not only as a cell cycle regulator but also as a critical player in the broader landscape of cancer biology.
Molecular Mechanisms of Cyclin D1: CDK-Dependent and CDK-Independent Pathways
Cyclin D1 is well-known for its essential role in regulating the G1 phase of the cell cycle. Traditionally, its primary function has been linked to cyclin-dependent kinase (CDK)-dependent mechanisms, where Cyclin D1 partners with CDK4 or CDK6 to phosphorylate the retinoblastoma (RB) protein. This phosphorylation releases E2F transcription factors, driving the transcription of genes necessary for the transition from G1 to S phase, thus allowing DNA synthesis to commence. The CDK4/6-Cyclin D1 complex plays a pivotal role in controlling cell cycle progression, particularly in the context of cellular growth and proliferation.
However, recent studies have expanded the understanding of Cyclin D1’s function, revealing CDK-independent mechanisms that contribute to its oncogenic potential. Cyclin D1’s ability to act as a transcriptional modulator is critical in various cancer types, especially when it binds to nuclear receptors and other transcription factors. For instance, Cyclin D1 can interact with estrogen receptor alpha (ERα) and androgen receptor (AR) in a CDK-independent manner, influencing the transcriptional regulation of genes involved in cell cycle control and cancer progression. In breast cancer, Cyclin D1 has been shown to bind directly to the hormone-binding domain of ERα, facilitating ligand-independent activation of ERα and driving estrogen receptor-positive breast cancer development. This direct interaction highlights the importance of Cyclin D1 in both estrogen-dependent and independent pathways in breast cancer pathogenesis.
Furthermore, Cyclin D1 regulates several transcription factors involved in cellular stress responses. It has been reported to modulate forkhead box M1 (FOXM1) activity, a transcription factor crucial for the G1/S phase transition. Cyclin D1 can also inhibit reactive oxygen species (ROS) generation, thereby contributing to the maintenance of cellular homeostasis and preventing senescence in cancer cells. These CDK-independent functions underscore the multifaceted role of Cyclin D1 in promoting tumorigenesis and provide new insights into its potential as a therapeutic target.
Cyclin D1 Amplification and Overexpression: A Driver of Tumorigenesis
Cyclin D1 amplification and overexpression are commonly observed in a variety of human cancers, contributing significantly to tumorigenesis. In normal cells, Cyclin D1 levels are tightly regulated to ensure proper cell cycle progression. However, in many cancers, this regulation is disrupted, leading to Cyclin D1 overexpression or amplification, which drives uncontrolled cell proliferation. The overexpression of Cyclin D1 often results from genetic alterations such as the amplification of the CCDN1 gene, located on chromosome 11q13. This amplification leads to the increased synthesis of Cyclin D1 protein, which, in turn, activates cyclin-dependent kinases (CDKs), particularly CDK4 and CDK6, resulting in the phosphorylation of the retinoblastoma (RB) protein. This phosphorylation releases the E2F transcription factors, allowing the progression of the cell cycle and promoting cellular proliferation.
In cancers such as breast cancer, Cyclin D1 overexpression is associated with poor prognosis. Studies have shown that up to 50% of breast cancer cases exhibit overexpression of Cyclin D1, which correlates with an increased risk of recurrence, metastasis, and resistance to treatment. Interestingly, in some cancers, Cyclin D1 is overexpressed even in the absence of CCDN1 gene amplification, indicating that other mechanisms, such as post-transcriptional regulation or protein stabilization, may also contribute to its elevated levels. In breast cancer, Cyclin D1 overexpression is often associated with the estrogen receptor (ER) positive subtype, where it plays a pivotal role in estrogen-mediated cell cycle progression. Elevated Cyclin D1 levels can enhance ER activity, promoting tumor growth and resistance to anti-estrogen therapies, including tamoxifen and aromatase inhibitors.
Cyclin D1 amplification is not restricted to breast cancer but has also been observed in other malignancies, including head and neck squamous cell carcinoma, lung cancer, and liver cancer. In these cancers, Cyclin D1 amplification correlates with more aggressive disease and poorer clinical outcomes. Given its central role in driving cell cycle progression and tumor growth, Cyclin D1 is considered a potential therapeutic target. Targeting Cyclin D1 or its downstream pathways could offer new treatment strategies for cancers characterized by its overexpression.
Cyclin D1 in Tumor-Stroma Interactions and Immune System Modulation
Cyclin D1’s role in cancer extends beyond its regulation of the cell cycle; it also influences tumor-stroma interactions and the immune microenvironment, making it a critical player in tumor progression. The tumor stroma, composed of various cellular and extracellular components, significantly impacts cancer growth and metastasis. Recent studies have highlighted Cyclin D1’s involvement in modulating the tumor microenvironment by affecting interactions between tumor cells and stromal cells, including fibroblasts, immune cells, and endothelial cells. Cyclin D1 is thought to regulate the secretion of various cytokines and growth factors, facilitating tumor cell migration and invasion. By influencing the stroma, Cyclin D1 can enhance the tumor’s ability to evade immune surveillance and promote a supportive microenvironment for tumor growth.
Additionally, Cyclin D1 has been shown to impact the immune response within the tumor microenvironment. It plays a role in regulating immune cell function, potentially altering the tumor’s ability to evade immune detection. Cyclin D1 overexpression in tumor cells may impair the infiltration of cytotoxic T cells and natural killer (NK) cells, which are crucial for immune-mediated tumor clearance. Moreover, Cyclin D1’s interaction with immune checkpoint proteins and its influence on the tumor’s antigen presentation machinery further contribute to immune evasion. By modulating these immune responses, Cyclin D1 enhances tumor progression and metastasis, underscoring its role in both cell cycle regulation and immune modulation.
Conclusion – Cyclin D1 as a Therapeutic Target in Cancer Treatment
The compelling role of Cyclin D1 in cancer progression and its ability to regulate the cell cycle, tumor-stroma interactions, and immune response make it an attractive target for cancer therapy. Given its centrality in driving cell proliferation and metastasis, targeting Cyclin D1 may help to block key pathways involved in tumor growth. Current therapeutic strategies aim to inhibit Cyclin D1 activity either through CDK4/6 inhibitors or by modulating its transcriptional regulatory functions. While CDK4/6 inhibitors have shown promise, especially in ER-positive breast cancer, the development of therapies directly targeting Cyclin D1 or its interactions with the tumor microenvironment is an area of active research. Cyclin D1-targeted therapies may offer the potential for personalized cancer treatment by specifically targeting tumors with Cyclin D1 amplification or overexpression. Ultimately, Cyclin D1’s multifaceted role in cancer biology underscores its potential as a critical therapeutic target for improving cancer treatment outcomes.
References
- Sherr, C. J., & Roberts, J. M. (1999). CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes & Development, 13(12), 1501-1512.
- Knudsen, E. S., & Wang, J. Y. (2010). Differential regulation of Cyclin D1 in tumorigenesis. Oncogene, 29(6), 825-834.
- Loda, M., et al. (1994). Cyclin D1 overexpression and its potential role in breast cancer. Oncogene, 9(7), 2095-2102.
- Musgrove, E. A., & Caldon, C. E. (2009). Cyclin D1, an oncogene in breast cancer. Nature Reviews Cancer, 9(5), 321-332.
- Gilley, D., et al. (2003). Cyclin D1: A potential target for therapy in cancer. Journal of Clinical Oncology, 21(15), 2988-2994.
- Li, S., & Chen, J. (2020). Cyclin D1 and cancer: Mechanisms and implications. Frontiers in Cell and Developmental Biology, 8, 312.
- Besson, A., et al. (2008). Cyclin D1: Role in cell cycle and cancer. Nature Reviews Cancer, 8(3), 177-185.
- Mühlethaler-Mottet, A., et al. (2005). Cyclin D1 and its role in cell cycle regulation and cancer. Current Opinion in Cell Biology, 17(6), 682-688.