Did You Know the Most Scented Chemical in The World?
Abstract
Fragrance is an essential aspect of our daily lives, and advancements in scientific research and technology have enabled us to delve deeper into the world of scents. This paper presents a compilation of some of the most fragrant chemicals, including vanillin, phenylethyl alcohol, cinnamaldehyde, menthol, and eugenol. These chemicals have a long and fascinating history and find extensive applications across various industries, including food, fragrance, medicine, and cosmetics. By comprehending the origins, properties, and development of these aroma chemicals, we gain a better understanding of the intricate nature of scent and its profound influence on our daily experiences. This knowledge also paves the way for innovative approaches in fragrance creation, product development, and the enhancement of olfactory encounters. With ongoing research in this field, we can anticipate further discoveries and a deeper comprehension of the captivating realm of fragrance.
Introduction
Scent plays a vital role in our daily lives. Since ancient times, people have been curious about the origins and properties of aromatic substances. We can now delve deeper into the most fragrant chemicals thanks to scientific advancements. Researchers employ advanced techniques like gas chromatography-mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) to identify and analyze fragrance substances. These methods help determine the chemical structure and composition, providing insights into their fragrance-producing mechanisms. Second, based on the existing fragrance database and big data analysis methods, researchers are conducting systematic research to discover and infer the most fragrant chemicals. By integrating and analyzing large amounts of scent data, scientists can identify compounds that share similar scent profiles and screen them for the most scented candidates. Furthermore, as olfactory perception is intensively studied, scientists are striving to understand the interaction between aroma substances and olfactory receptors. Researchers have revealed the binding mechanism and signal transduction pathway between olfactory receptors and aroma substances through structural biology, computational simulation, and functional studies. These studies help us understand why certain compounds have strong fragrance properties. Therefore, if you want to understand the most fragrant chemicals in the world, you must study the basic knowledge and development of these compounds. Here is the list of the most fragrant chemicals in the world!
Vanillin
Vanillin, known for its distinct vanilla scent, was initially extracted from vanilla bean pods, hence its name. Throughout history, the unique aroma substance found in vanilla pods was identified as vanillin. Originally, vanillin was obtained through a time-consuming and expensive process of solvent extraction and concentration from vanilla pods. However, as chemical technology advanced, the synthesis of vanillin became a viable solution to meet the increasing demand. In the late 19th century, German chemist Ferdinand Mond achieved a groundbreaking milestone by synthesizing the first artificial version of vanillin, enabling large-scale production. As researchers delved deeper into vanillin, they explored its chemical properties, biosynthetic pathways, and applications. They conducted studies on its structure, properties, and aroma characteristics, aiming to discover more efficient synthesis methods. Vanillin finds widespread use in the food, perfume, and medicine industries. In the food industry, it serves as a flavoring agent, imparting a robust vanilla taste to various food products. Additionally, vanillin is a commonly employed ingredient in perfumes, lending a sweet and warm aroma to fragrance compositions.Phenethyl alcohol
Phenethyl alcohol (C8H10O) is an organic compound renowned for its floral fragrance. It naturally occurs in plants and flowers, particularly in notable amounts within roses, jasmine, and carnations. Its distinctive floral scent has led to its widespread use in perfumes, cosmetics, fragrances, and essences. Initially, phenethyl alcohol was extracted from roses in the 19th century for use in rose perfumes. However, the expensive extraction process prompted the development of synthetic methods to make its production and application more accessible. As the perfume and fragrance industry progressed, phenethyl alcohol became widely utilized in perfumes, bath products, skincare items, cosmetics, and even as a food flavoring agent to impart a floral taste to culinary delights. Advancements in modern chemical technology have enabled more efficient and sustainable production of phenethyl alcohol. It can now be synthesized through methods like plant extraction, chemical synthesis, or biological fermentation. In conclusion, phenethyl alcohol, with its floral aroma, plays a significant role in industries such as perfume, fragrance, and cosmetics. It's rich history and versatile applications make it a favored choice for those seeking aromatic experiences.Cinnamaldehyde
Cinnamaldehyde (C9H8O) is an organic compound derived from the bark of the cinnamon tree, serving as one of the primary aroma components of cinnamon. It possesses a distinctive spicy, warm, and sweet scent, making it a vital ingredient in various products, including spices, food additives, and flavors. Cinnamaldehyde has a long history of use, with cinnamon being employed in plasters and spices since ancient Egypt dating back to 2800 BC. Cinnamon bark was extensively utilized in food flavoring and herbal medicine in ancient China, India, and the Middle East. Over time, extraction and synthesis methods for cinnamaldehyde have improved and evolved. The common extraction method involves distilling or extracting the bark of the cinnamon tree to obtain higher-purity cinnamaldehyde. Additionally, chemical synthesis methods are widely used to produce cinnamaldehyde through chemical reactions involving other raw materials. As the food and fragrance industries have advanced, the applications of cinnamaldehyde have expanded. It is extensively employed in food flavorings, baked goods, desserts, chewing gum, and oral care products, providing them with their distinctive cinnamon aroma. Moreover, cinnamaldehyde finds use in the production of perfumes, essences, soaps, and cosmetics. Modern technology has facilitated the more efficient and sustainable production of cinnamaldehyde. By enhancing extraction and synthesis methods, cinnamaldehyde can be manufactured on a large scale to meet market demands. In summary, cinnamaldehyde, with its pungent, warm, and sweet aroma, plays a crucial role in the food and fragrance industries. Its rich historical background and versatile applications establish cinnamaldehyde as a beloved spice.Menthol
Menthol (C10H20O) is an organic compound that can be extracted naturally from the leaves and stems of the peppermint plant or produced synthetically. Known for its refreshing, mint-like aroma and taste, menthol finds diverse applications in medicine, food, oral care products, and flavors. Its usage dates back to ancient times, with mint plants being utilized in herbal medicine and spice production in ancient Egypt and Greece. Traditional healers and herbalists often employed peppermint to address issues like colds, digestive problems, and skin ailments. Research and development surrounding menthol have progressed over time. By the late 19th century, extraction and purification methods for menthol had been refined, enabling large-scale production. Peppermint oil, rich in menthol, became widely used in the pharmaceutical, oral care, and cigarette industries, providing a cooling sensation and distinct aroma to these products. Alongside the natural source, menthol can also be synthesized through chemical processes. Synthetic methods for menthol emerged in the 20th century, resulting in more sustainable and cost-effective production. Menthol boasts a broad range of applications. It is frequently employed in pharmaceuticals and medical supplies such as mouth fresheners, pain balms, cold remedies, anti-itch treatments, and skincare products. In the food and beverage industry, menthol serves as a flavoring agent, imparting a cooling taste and aroma. Additionally, menthol contributes to the production of perfumes, essences, soaps, and cosmetics. Overall, menthol possesses a rich historical background and finds extensive use as a chemical compound with a cooling, minty flavor. Its distinct aroma and versatility make it an indispensable ingredient in fields such as medicine, food, and cosmetics.Eugenol
Eugenol (C10H12O2) is a natural organic compound extracted from the buds and leaves of the clove tree (Syzygium aromaticum). It serves as the primary ingredient in cloves and possesses a robust fragrance and spicy taste. Eugenol finds extensive use in various domains, including medicine, food, spices, and cosmetics. The historical applications and research surrounding eugenol span many centuries. Ancient civilizations' texts mention the medicinal uses of the clove plant, while in medieval Europe, eugenol was utilized as an herbal remedy for toothaches, digestive discomfort, and other health issues. Additionally, eugenol adds flavor to food as a preservative and spice. Advances in science and technology have deepened our understanding of eugenol. Modern studies have revealed its diverse biological activities, including antibacterial, antioxidant, anti-inflammatory, and analgesic effects. This makes eugenol an important component in pharmaceuticals and oral care products. In the food and beverage industry, eugenol serves as a flavoring agent, imparting a distinctive aroma to various products, particularly meat, pastries, and desserts. Furthermore, eugenol plays a vital role in the manufacturing of perfumes and cosmetics. It is used to create products like perfumes, soaps, toothpaste, and chewing gum, providing them with a refreshing scent and taste. In summary, eugenol, as a natural organic compound, boasts a rich historical background and wide-ranging applications. Its herbal properties and versatility make it a significant contributor to fields such as medicine, food, fragrance, and cosmetics.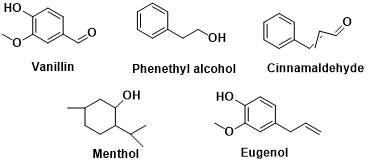
Figure 1: Structural formula
References
- Ma Q, Liu L, Zhao S, Huang Z, Li C, Jiang S, Li Q, Gu P. Biosynthesis of vanillin by different microorganisms: a review. World J Microbiol Biotechnol. 2022 Jan 12;38(3):40. doi: 10.1007/s11274-022-03228-1. PMID: 35018518.
- Moradi O. A review on nanomaterial-based electrochemical sensors for determination of vanillin in food samples. Food Chem Toxicol. 2022 Oct;168:113391. doi: 10.1016/j.fct.2022.113391. Epub 2022 Aug 28. PMID: 36041662.
- Tian XY, Li MX, Lin T, Qiu Y, Zhu YT, Li XL, Tao WD, Wang P, Ren XX, Chen LP. A review on the structure and pharmacological activity of phenylethanoid glycosides. Eur J Med Chem. 2021 Jan 1;209:112563. doi: 10.1016/j.ejmech.2020.112563. Epub 2020 Aug 28. PMID: 33038797.
- Doyle AA, Stephens JC. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia. 2019 Nov;139:104405. doi: 10.1016/j.fitote.2019.104405. Epub 2019 Nov 7. PMID: 31707126.
- Muhoza B, Qi B, Harindintwali JD, Koko MYF, Zhang S, Li Y. Encapsulation of cinnamaldehyde: an insight on delivery systems and food applications. Crit Rev Food Sci Nutr. 2023;63(15):2521-2543. doi: 10.1080/10408398.2021.1977236. Epub 2021 Sep 13. PMID: 34515594.
- Zhao Y, Pan H, Liu W, Liu E, Pang Y, Gao H, He Q, Liao W, Yao Y, Zeng J, Guo J. Menthol: An underestimated anticancer agent. Front Pharmacol. 2023 Mar 17;14:1148790. doi: 10.3389/fphar.2023.1148790. PMID: 37007039; PMCID: PMC10063798.
- Silva H. Current Knowledge on the Vascular Effects of Menthol. Front Physiol. 2020 Apr 7;11:298. doi: 10.3389/fphys.2020.00298. Erratum in: Front Physiol. 2020 Oct 20;11:602231. PMID: 32317987; PMCID: PMC7154148.
- Taleuzzaman M, Jain P, Verma R, Iqbal Z, Mirza MA. Eugenol as a Potential Drug Candidate: A Review. Curr Top Med Chem. 2021;21(20):1804-1815. doi: 10.2174/1568026621666210701141433. PMID: 34218781.
- Zari AT, Zari TA, Hakeem KR. Anticancer Properties of Eugenol: A Review. Molecules. 2021 Dec 6;26(23):7407. doi: 10.3390/molecules26237407. PMID: 34885992; PMCID: PMC8659182.