Discovery and Summary of New Targets for Cancer Immunity
Abstract
Optimized immunotherapy has completely changed cancer treatment by using the body ‘s immune system to attack cancer cells. The key to this method is to identify immune targets, that is, molecules on cancer cells can be recognized by immune cells. Immune checkpoint targets, such as PD-1 and CTLA-4, have been successfully targeted to checkpoint inhibitors to enhance anti-tumor immunity. However, many patients do not respond to checkpoint inhibitors, highlighting the need for new targets. Recently, the discovery of other promising targets including tumor-associated macrophages and myeloid-derived suppressor cells has provided a new way to develop effective tumor immunotherapy.
What are immunotherapy and the mechanism of immunotherapy ?
Immunotherapy is a type of cancer treatment that uses the patient’s immune system to fight cancer cells. It works by stimulating the immune system to attack cancer cells or by using immune cells or substances to target cancer cells directly. Immunotherapy can be used alone or in combination with other cancer treatments such as chemotherapy or radiation therapy.
There are several different types of immunotherapy, including:
- Checkpoint inhibitors: These drugs block proteins called checkpoints on immune cells that prevent them from attacking cancer cells.
- Adoptive cell transfer: This treatment involves removing T cells (a type of immune cell) from a patient’s body, modifying them in a laboratory to better recognize and attack cancer cells, and then infusing them back into the patient’s body.
- Monoclonal antibodies: These are lab-made versions of immune system proteins that can be designed to target specific parts of cancer cells.
- Cancer vaccines: These vaccines can help stimulate the immune system to recognize and attack cancer cells.
- Immune system modulators: These drugs can help boost or suppress the immune system to better fight cancer.
- Oncolytic virus therapy: This approach involves using viruses that can selectively infect and kill cancer cells while sparing normal cells.
- CAR-T cell therapy: This treatment involves modifying a patient’s own T cells to express chimeric antigen receptors (CARs) that recognize and attack cancer cells.
Immunotherapy has shown promise in treating several types of cancer, include molanoma, lung cancer ,and bladder cancer,among others. Howeverr, it is not effective for all patients, and some patients may experience side effects from immunotherapy.
What are Checkpoint inhibitors?
Checkpoint inhibitors have brought about a revolutionary change in cancer treatment in recent years. They belong to the class of cancer immunotherapy drugs that operate by inhibiting specific checkpoints on immune cells that hinder them from attacking cancer cells. By dismantling these obstacles, checkpoint inhibitors can boost the immune system’s ability to detect and destroy cancer cells with greater effectiveness.
Pembrolizumab, one of the most extensively utilized checkpoint inhibitors, focuses on the PD-1 receptor present on immune cells. It has proven to be effective in the treatment of diverse cancers, including melanoma, bladder cancer, and non-small cell lung cancer. Another commonly used checkpoint inhibitor, ipilimumab, targets the CTLA-4 receptor on immune cells and is frequently used in conjunction with other checkpoint inhibitors for enhanced effectiveness against melanoma.
Compared to traditional cancer therapies such as chemotherapy and radiation therapy, checkpoint inhibitors offer numerous advantages. They have fewer side effects and are less toxic. Additionally, since they operate by amplifying the body’s immune system, checkpoint inhibitors have the potential to provide long-term protection against cancer recurrence.
While checkpoint inhibitors have advantages in cancer treatment, they are not a one-size-fits-all solution. Effectiveness varies among patients and they can be expensive. Additionally, the immune system may attack healthy tissues, causing autoimmune reactions. It’s crucial for doctors and patients to be aware of these potential side effects and monitor patients closely for any adverse reactions.
Checkpoint inhibitors are a significant breakthrough in cancer treatment, holding the potential to greatly improve the prognosis for cancer patients. Ongoing research is dedicated to developing new inhibitors and enhancing the effectiveness of existing treatments. Continued advancements in this field offer hope that checkpoint inhibitors will become an increasingly crucial weapon in the battle against cancer.
Mechanism of immune checkpoints
Immune checkpoints are a class of immunoregulatory molecules that can either stimulate or inhibit immune responses. They play an important role in maintaining immune homeostasis and preventing autoimmune responses. However, cancer cells can exploit these checkpoints to evade the immune system and promote their growth and survival.
For example, there are now a group of drug dealers who need to go through customs security inspections. The drug dealers are similar to antigen-presenting cells (such as tumor cells, etc.), the security inspection system is similar to the human immune system, and the security personnel are similar to T cells in the immune system. At that time, if a drug trafficker wants not to be reported by the security inspectors, he can find connections and spend money to bribe the security inspectors. At this time, if the security inspectors have a greedy desire and will have an evil intention to restrain their duties, then they will accept money from drug dealers , and will not report the drug dealer when he goes through the security check (it can be identified, but not killed), and he will be released as an ordinary citizen (normal cell). At this time, the evil thoughts of the security personnel are similar to the immune checkpoint. Drug dealers use this mechanism to suppress security personnel, escape from the customs security system and survive. Immune checkpoint inhibitor drugs are similar to the ICAC, which can relieve this inhibitory effect and allow security personnel to reactivate their work. Identify and notify the public security organs to arrest and eliminate drug dealers.
The immune checkpoint is like a checkpoint, telling the immune system whether to continue killing or “rest from get off work”. Tumor cells take advantage of the command function of immune checkpoints, so that the body’s immune system is always in a state of “off work” and cannot work normally.
The mechanism of immune checkpoint inhibitors is to promote the activation and proliferation of T cells by inhibiting immune checkpoints and relieving the immunosuppression of tumor cells on T cell activation, thereby killing tumor cells.
At present, the widely used ICIs mainly include CTLA-4 inhibitors, PD-1 inhibitors and PD-L1 inhibitors.
The structure, function and mechanism of programmed cell death protein 1 ( PD-1 ) and programmed death receptor ligand 1 ( PD-L1 ) :
PD-1, also called CD279, is a type I transmembrane protein receptor with an extracellular domain that has a typical Ig-like structure. It plays a crucial role in mediating cell-cell interactions and signaling. PD-1 has two natural ligands, PD-L1 (also known as B7-H1 and CD274) and PD-L2 (also known as B7-DC and CD273). Upon binding to PD-L1/PD-L2, PD-1 becomes phosphorylated and recruits protein tyrosine phosphatase 2, which dephosphorylates several key molecules in the T cell receptor signaling pathway. This process inhibits T cell proliferation and activity, allowing tumor cells to grow.
The first anti-PD-1 antibodies approved for clinical use are Nivolumab (Bristol Myers Squibb, USA) and Pembrolizumab (Merck, USA).
Currently, the potential mechanism for immune-related adverse events (irAEs) induced by anti-PD-1 or anti-PD-L1 antibodies involves the overactivation of effector T cells and the decreased function of regulatory T cells caused by the inhibition of the PD-1/PD-L1 pathway. This process activates macrophages and neutrophils, leading to increased tumor toxicity, massive release of interferon-gamma and tumor necrosis factor, and antibody production by B cells.
The structure, function and mechanism of cytotoxic T lymphocyte-associated antigen 4 ( CTLA-4 ) :
CTLA-4, also known as CD152, is a type I transmembrane glycoprotein belonging to the immunoglobulin superfamily. It is composed of 223 amino acids and exists as a covalent homodimer with a molecular weight of 41-43 kDa. Its extracellular domain consists of an immunoglobulin-like type V domain that houses the B7-1 (CD80)/B7-2 (CD86) ligand-binding site. CTLA-4 is expressed on regulatory T cells and activated T cells as an ICs receptor, and it mainly serves an immunosuppressive function in the early stages of T cell activation within lymphoid tissues. CTLA-4 can outcompete CD28, a co-stimulatory signal molecule expressed on the surface of T cells, for the same ligand. Both CTLA-4 and CD28 share a similar structure and function and bind to two B7 family members expressed on antigen-presenting cells through the MYPPPY motif (CD80 and CD86).
Ipilimumab (Bristol Myers Squibb, USA), a humanized monoclonal antibody, is currently the immune checkpoint inhibitor developed for CTLA-4. It blocks the CTLA-4-B7 pathway, promotes the proliferation and activation of effector T cells, and enhances the anti-tumor immune response, leading to the eradication of tumor cells. However, anti-CTLA-4 treatment may result in the non-specific activation of the immune system, causing immune-related adverse events (irAEs).
In addition to the above CTLA-4, PD-1, PD-L1 antibodies, there are more target antibodies in the study of immune checkpoint inhibitors :
LAG-3
LAG-3, also known as Lymphocyte-activation gene 3, is a protein that is present on the surface of specific immune cells, such as T cells and natural killer (NK) cells. Its primary role is to regulate the immune response and manage the activity of T cells and NK cells. As a negative regulator of T cell function, LAG-3 has the ability to dampen down or suppress the immune response.
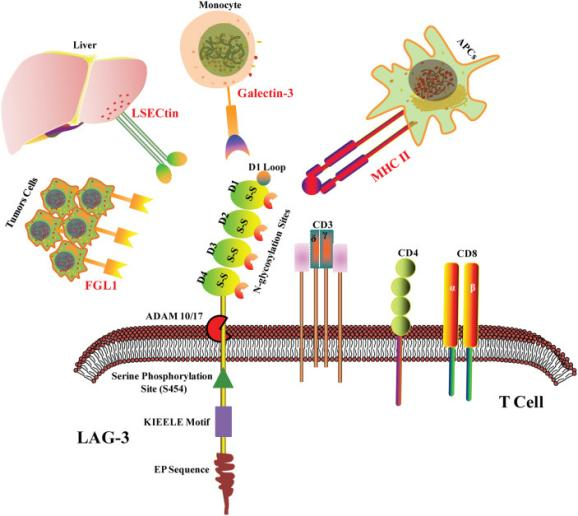
Figure 1 LAG-3 structure and ligands.
Studies have demonstrated the importance of LAG-3 in the progression of autoimmune diseases and cancer. By targeting LAG-3, it is possible to either activate the immune response against cancer cells or suppress the immune response in autoimmune diseases.
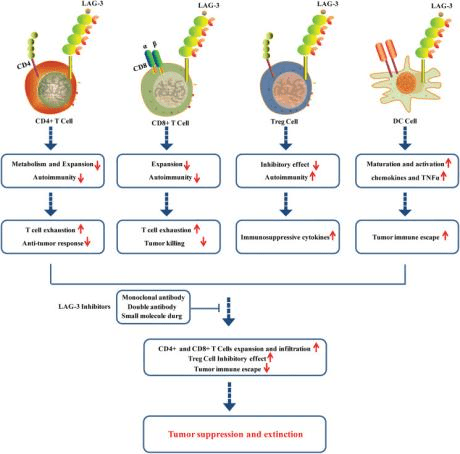
Figure 2 Roles of LAG-3 in CD+4 cells, CD8+ cells, Treg cells and DC cells in tumor microenvironment.
Numerous drugs that target LAG-3 are currently under development as standalone therapies or in combination with other immunotherapies. Clinical trials are ongoing to evaluate these drugs’ efficacy in treating a variety of conditions, such as melanoma, lung cancer, and autoimmune disorders like rheumatoid arthritis.
In summary, LAG-3 presents a promising target for developing new immunotherapies capable of manipulating the immune response and treating a broad range of diseases.
TIM-3
- cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) is a protein expressed on the surface of various immune cells, including T cells, natural killer (NK) cells, and dendritic cells. TIM-3 plays a critical role in regulating immune responses and has been found to be involved in various diseases such as autoimmune disorders, infectious diseases, and cancer. It acts as a negative regulator of immune responses by binding to specific ligands on target cells, which results in immune suppression.
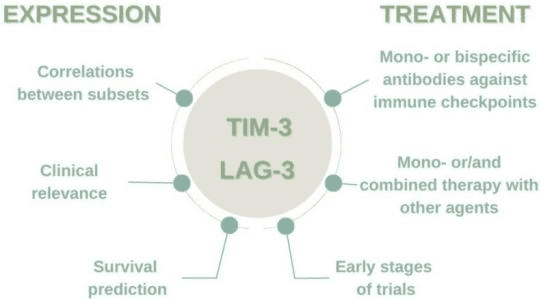
Figure 3 The importance of TIM-3 and LAG-3 expression in the microenvironment and immunotherapy.
In cancer, TIM-3 plays a crucial role in the tumor’s immune evasion by inhibiting the activity of tumor-infiltrating immune cells. This mechanism contributes to the resistance of tumor cells to immune-based therapies such as checkpoint inhibitors. Consequently, TIM-3 has gained significant interest as a potential target for cancer immunotherapy. Several TIM-3 inhibitors are currently undergoing clinical trials to evaluate their safety and efficacy in treating various cancers. TIM-3 is a promising target in cancer immunotherapy that has the potential to enhance the effectiveness of immune-based treatments and improve patient outcomes.
TIGIT
T-cell immunoreceptor with Ig and ITIM domains (TIGIT) is an immune cell surface protein expressed in T cells, NK cells, and some dendritic cells. Belonging to the CD28 receptor family, TIGIT interacts with multiple ligands, including CD155 and CD112, and regulates immune responses critical in various diseases, such as autoimmune disorders, infectious diseases, and cancer.
TIGIT inhibits T cell and NK cell activation and promotes regulatory immune cell development, thereby suppressing immune responses. In cancer, TIGIT contributes to immune evasion by suppressing tumor-infiltrating immune cells’ activity, similar to TIM-3. This mechanism leads to tumor cells’ resistance to immune-based therapies like checkpoint inhibitors.
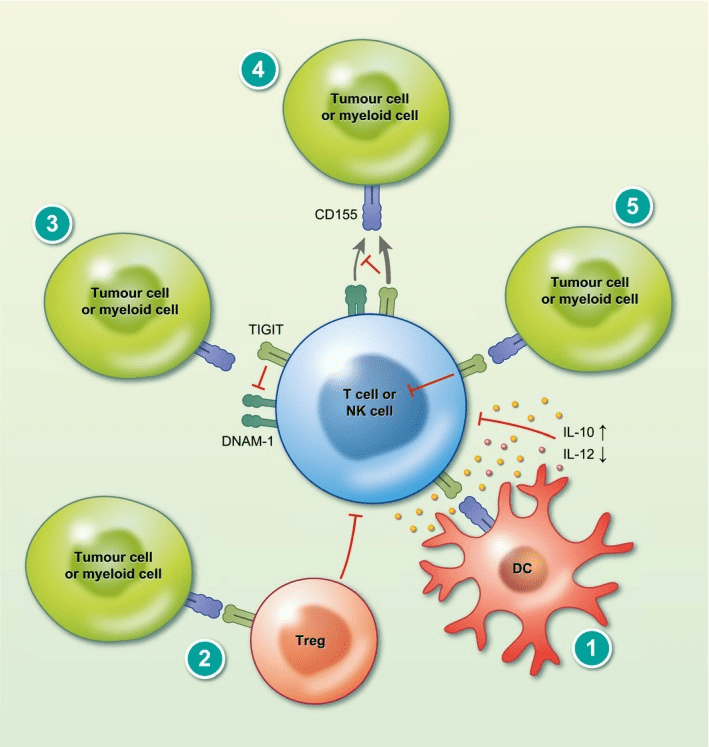
Figure 4 T cell immunoglobulin and ITIM domain (TIGIT) mechanism of action.
Therefore, TIGIT has gained significant attention as a potential target for cancer immunotherapy, and several TIGIT inhibitors are undergoing clinical trials to evaluate their safety and efficacy in treating various cancers.
V-Domain Immunoglobulin-Containing Suppressor of T Cell Activation
VISTA, an immunoglobulin-containing suppressor of T cell activation, is a crucial protein involved in regulating the immune system. Mainly expressed on immune cells like T cells, myeloid cells, and dendritic cells, it belongs to the immunoglobulin superfamily.
Discovered in 2011, VISTA plays a negative role in T cell activation by inhibiting cytokine production and T cell proliferation. By doing so, it prevents excessive immune responses that could harm healthy tissues. VISTA acts as a checkpoint inhibitor, like PD-1 and CTLA-4, by suppressing T cell activation and ensuring immune homeostasis when it binds to its ligand.
The identification of VISTA has brought new possibilities in cancer immunotherapy. Inhibiting VISTA enhances T cell activation and boosts anti-tumor immunity. Recent preclinical studies have shown that VISTA blockade can improve the effectiveness of other checkpoint inhibitors, such as anti-PD-1 antibodies, in cancer treatment.
Clinical trials are presently ongoing to assess the safety and efficacy of VISTA-targeted therapies in humans. The scientific community has shown significant interest in using VISTA as a therapeutic target for cancer, and further research is necessary to comprehend its immune system functions and its potential in immunotherapy.
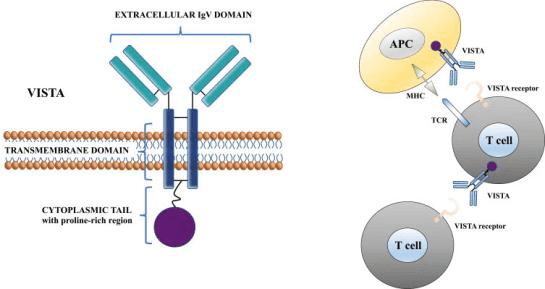
Figure 5 Molecular structure of VISTA protein and its function at a cellular level.
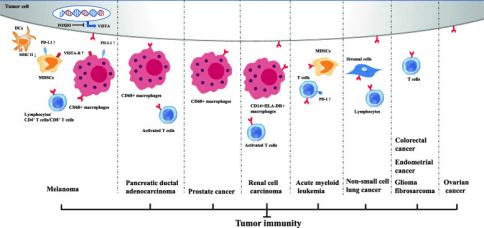
Figure 6 Inhibitory immune checkpoint roles of VISTA in anti-cancer immunity. Positive expression of VISTA on tumor cells and/or immune cells induces an immunosuppressive environment in multiple cancer types
B and T lymphocyte attenuator (BTLA)
B and T lymphocyte attenuator (BTLA) is a cell surface protein that is expressed on various immune cells, including T cells, B cells, and natural killer (NK) cells. BTLA is a member of the immunoglobulin superfamily and functions as an inhibitory immune checkpoint receptor that regulates immune responses.
BTLA interacts with its ligand, herpesvirus entry mediator (HVEM), which is expressed on antigen-presenting cells, including dendritic cells and macrophages. Upon engagement with HVEM, BTLA delivers inhibitory signals to T cells, leading to the suppression of T cell activation and proliferation. BTLA also regulates B cell function by inhibiting B cell activation and antibody production.
Studies have shown that the expression of BTLA is upregulated in various types of cancer, including melanoma, lung cancer, and bladder cancer. In cancer, BTLA can contribute to the suppression of anti-tumor immune responses by inhibiting the activation of T cells and other immune cells. Therefore, blocking BTLA with antibodies or small molecules has emerged as a potential strategy for cancer immunotherapy.Preclinical studies have shown that blockade of BTLA can enhance anti-tumor immunity and improve the efficacy of cancer immunotherapy in mouse models.
In recent years, other immune checkpoint new target molecules have also received attention. In addition, more new tumor immune targets have been discovered.
Immune checkpoint blocking new targets
New targets of B7 family
- VSIG4 ( V-set and immunoglobulin domain-containing protein 4 ) inhibits the cytotoxicity of CD8 + T cells after binding to its ligands and is another potential target for tumor immunosuppression in the B7 family. VSIG4-specific antibodies showed the ability to re-polarize macrophages and induce immune activation in both in vitro and in vivo models, and tumor growth inhibition was observed in mouse models with VSIG4 antibody alone and in combination with anti-PD-1.
- B7-H3 ( CD276 ), a member of the B7 immunomodulatory molecule family, is overexpressed in primary and metastatic prostate cancer and is associated with disease severity and poor clinical outcomes. MGC018 is a B7-H3 antibody drug conjugate based on double-cardomycin, which shows strong anti-tumor activity against CDX and PDX models of prostate cancer in vivo.
- BTN3A1 is a member of the B7 costimulatory receptor family, mediating Vγ9Vδ2 T cells to recognize phosphoantigen ( PAg ), which is expected to become a new target for tumor immunotherapy. BTN3 A monoclonal antibody developed by ImCheck Therapeutics activates Vγ9Vδ2 T cells in the blood circulation and kills a variety of tumor cells. The current clinical data have shown good therapeutic effects.
New targets for tumor immunosuppression
- GPR65 ( G protein-coupled receptor 65 ) is a new innate immune checkpoint that affects the function of tumor microenvironment ( TME ) and tumor-associated macrophages ( TAM ). Inhibition of GPR65 is a new method for cancer treatment. GPR65 small molecule inhibition of PTT-3213 can significantly increase CD8 + T cells and natural killer T cells in the tumor microenvironment, and cooperate with PD1 antibody to produce better efficacy in the mouse MC38 tumor model.
- The release of GABA (gamma-aminobutyric acid metabolite) by B cells has been discovered to stimulate the differentiation of M2 macrophages, suppress the cytotoxic response of CD8+ T cells in tumors, and offer a fresh avenue for researching tumor immunotherapy targets.
- DDR1 (discoid domain receptor 1) can prevent immune cells from approaching tumors, and inhibiting the expression of DDR1 can restore the immune response in TME, which points to a new direction for tumor immunotherapy.
- ART1 (ADP-ribosyltransferase 1) was found to block anti-tumor immune cells when expressed on tumor cells. In animal models of cancer, blocking ART1 increased the presence of anti-tumor immune cells in the TME and slowed or halted tumor progression. growth, revealing another new target in tumor therapy.
- ARIH1 (Aradne RBR E3 ubiquitin protein ligase 1) was identified as a new E3 ubiquitin ligase of PD-L1, and the latest research results found that cancer escapes immunity through EGFR-GSK3α-ARIH1 signaling, suggesting that ARIH1 may be the key to enhance anti-tumor immunity new targets for therapy.
- CD155 (poliovirus receptor) is a key regulator of immune activation, and increased expression levels are associated with resistance to anti-PD-(L)1 therapy, while loss of CD155 leads to reduced tumor growth, which is expected to be a promising candidate for tumor immunotherapy. Novel target. CD155 antibody NTX-1088 has obvious advantages in activating T and NK cells, and exhibits strong anti-tumor effect.
- CD200R1 (cell surface transmembrane glycoprotein) inhibits immune cell activity after binding to its ligand, which may be a key checkpoint for cancer immune surveillance and a promising therapeutic target. Humanized monoclonal antibody 23ME-00610 may reverse CD200-mediated immunosuppression in the TME and enhance antitumor T cell function in cancer patients.
- LIF (leukemia inhibitory factor), an immunosuppressive cytokine associated with tumor growth and metastasis, hinders the recruitment of cytotoxic CD8+ T cells in the TME. LIF overexpression is associated with poor prognosis and chemotherapy resistance in a variety of cancers. Monotherapy with the humanized LIF monoclonal antibody AZD0171 demonstrated a manageable safety profile and prevented cancer progression in 34.2% of patients with advanced solid tumors.
CD161 is a KLRB1 gene-edited C-type lectin-like membrane receptor that inhibits the anticancer activity of immune cells, KLRB1 gene inactivation or antibody-mediated CD161 blockade can enhance T cell-mediated killing of glioma cells in vitro and in vivo Anti-tumor function, suggesting that CD161 may be a new target for tumor immunotherapy.
Amplification of SETDB1 (SET-domain-forked histone lysine methyltransferase 1) in human tumors is associated with immune rejection and resistance to immune checkpoint blockade, and SETDB1 deletion triggers specific cytotoxic T cells in vivo response, indicating that SETDB1 is an epigenetic checkpoint that suppresses tumor-intrinsic immunogenicity and is a candidate new target for tumor immunotherapy.
New target for specific cancer therapy
- Deletion of COP1 (E3 ubiquitin ligase) in cancer cells results in decreased macrophage-associated chemokine secretion and tumor macrophage infiltration, and increases tumor response to immune checkpoint inhibitor therapy, revealing that COP1 may be New targets for immunotherapy in triple-negative breast cancer.
- The fusion protein CLIP1-LTK, which combines microtubule end tracking protein and receptor tyrosine kinase, has been identified as a novel target for treating non-small cell lung cancer. Administration of the inhibitor lorlatinib to patients carrying CLIP1-LTK resulted in favorable clinical outcomes.
- LSD1 (lysine demethylase 1) was the first reported histone demethylase. Targeting LSD1 sensitizes small cell lung cancer (SCLC) to MHC-I-restricted T cell-dependent cytolysis and enhances immune checkpoint blockade efficacy in refractory SCLC tumors. These studies reveal that LSD1 may be a new target for SCLC immunotherapy.
- A small molecule drug called STM2457 targets METTL3, a methyltransferase-like protein, and inhibits the growth and spread of leukemia cells in a mouse model of acute myeloid leukemia (AML). This drug significantly extends the lifespan of the mice, offering a novel approach to treating AML.
- CD161 was found to be expressed on T cells of glioblastoma and inhibited the anti-cancer activity of T cells, suggesting that CD161 may be a new target for immunotherapy of malignant brain tumors.
- DDR1 (discoid domain receptor 1) is a newly discovered tyrosine protein kinase receptor involved in the progression of breast cancer and other tumors. Triple-negative breast cancer mouse models with DDR1 knockout or DDR1 antibody treatment both increased intratumoral T cell infiltration and inhibited tumor growth. This reveals that DDR1 is a potential new target for tumor immunotherapy.
- BCAM (basal cell adhesion molecule) is a member of the immunoglobulin superfamily that promotes cell adhesion, migration and invasion, is overexpressed in epithelial cancers such as skin cancer, and is involved in tumor progression. The antibody-drug conjugate of BCAM, GENA-111-auristatin F ADC, can significantly reduce the growth of tumor cells, so BCAM-targeted ADCs may be a promising therapeutic strategy for patients with BCAM-positive epithelial cancer.
- NSD3 (nuclear receptor-binding SET domain protein 3), a histone-lysine N-methyltransferase, is aberrantly expressed in lung squamous cell carcinoma (LUSC), and in tumors with NSD3 hyperactivity, Elevated H3K36 dimethylation accelerated tumorigenesis and decreased overall survival in a mouse model of LUSC, suggesting that NSD3 may be a novel epigenetic target in lung squamous cell carcinoma.
More new targets for tumor immunity
- IL-8 (interleukin 8) is involved in inflammation and immune defense in the body. Most tumors can secrete IL-8 to promote their own growth and participate in the formation of tumor TME. BMS-986253 (a fully human IgG1 anti-IL-8 monoclonal antibody) combined with anti-PD-1 nivolumab was well tolerated, had no dose-limiting toxicity, and showed preliminary antitumor activity.
- ID3 and SOX4 (inhibitor of cell differentiation, SRY-Box transcription factor 4) are genetic regulators that play a key role in the process of T cell exhaustion, and silencing these two factors greatly improves the effect of CAR-T on killing tumors. New avenues for cancer treatment.
- ELANE (neutrophil elastase) is a protease secreted by neutrophils that can kill a variety of cancer cells but has no harm to non-cancer cells. It can inhibit the growth of primary tumors and produce CD8+ T cell mediators. The induced distant effect prevents distant metastasis, which provides a new target for anticancer therapy.
The area of tumor immunotherapy is progressing swiftly, thanks to the emergence of drugs targeting PD-1/PD-L1 which are driving the discovery of fresh targets to enhance tumor immunity. In addition to immune checkpoint molecules, recent breakthroughs over the past two years have revealed further targets connected to immunology and epigenetics. These thrilling findings and ongoing advancements in identifying new targets for tumor immunity have the potential to offer groundbreaking solutions for treating tumors.
Conclusion
Over the past few years, significant progress has been made in identifying immune targets for the treatment of cancer. Immunotherapy, a cancer treatment that mobilizes the patient’s immune system to attack cancer cells, offers a major advantage in that it can selectively target cancer cells without harming healthy cells.
One class of immune targets, known as immune checkpoint proteins, regulates the immune response. Unfortunately, cancer cells can hijack these proteins to evade detection by the immune system. However, drugs that target immune checkpoint proteins, like PD-1 and CTLA-4, have shown remarkable success in treating multiple types of cancer.
In addition to immune checkpoint targets, researchers have identified other promising targets for cancer treatment, including neoantigens – unique proteins produced by cancer cells that can be recognized by the immune system and targeted by immunotherapy.
Overall, these advances in cancer immunotherapy hold great promise for improving cancer treatment outcomes. By targeting immune checkpoint proteins, neoantigens, and using therapies like CAR T-cell therapy, we are making strides towards more effective and personalized cancer treatments.
References
- Shan C, Li X, Zhang J. Progress of immune checkpoint LAG-3 in immunotherapy. Oncol Lett. 2020 Nov;20(5):207. doi: 10.3892/ol.2020.12070. Epub 2020 Sep 8. PMID: 32963613; PMCID: PMC7491111.
- Huo JL, Wang YT, Fu WJ, Lu N, Liu ZS. The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front Immunol. 2022 Jul 26;13:956090. doi: 10.3389/fimmu.2022.956090. PMID: 35958563; PMCID: PMC9361790.
- Sauer N, Szlasa W, Jonderko L, Oślizło M, Kunachowicz D, Kulbacka J, Karłowicz-Bodalska K. LAG-3 as a Potent Target for Novel Anticancer Therapies of a Wide Range of Tumors. Int J Mol Sci. 2022 Sep 1;23(17):9958. doi: 10.3390/ijms23179958. PMID: 36077354; PMCID: PMC9456311.
- Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016 May 17;44(5):989-1004. doi: 10.1016/j.immuni.2016.05.001. PMID: 27192565; PMCID: PMC4942846.
- Kozłowski M, Borzyszkowska D, Cymbaluk-Płoska A. The Role of TIM-3 and LAG-3 in the Microenvironment and Immunotherapy of Ovarian Cancer. Biomedicines. 2022 Nov 5;10(11):2826. doi: 10.3390/biomedicines10112826. PMID: 36359346; PMCID: PMC9687228.
- Zhao L, Cheng S, Fan L, Zhang B, Xu S. TIM-3: An update on immunotherapy. Int Immunopharmacol. 2021 Oct;99:107933. doi: 10.1016/j.intimp.2021.107933. Epub 2021 Jul 2. PMID: 34224993.
- Yue C, Gao S, Li S, Xing Z, Qian H, Hu Y, Wang W, Hua C. TIGIT as a Promising Therapeutic Target in Autoimmune Diseases. Front Immunol. 2022 Jun 3;13:911919. doi: 10.3389/fimmu.2022.911919. PMID: 35720417; PMCID: PMC9203892.
- Harjunpää H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020 May;200(2):108-119. doi: 10.1111/cei.13407. Epub 2019 Dec 25. PMID: 31828774; PMCID: PMC7160651.
- Tagliamento M, Agostinetto E, Borea R, Brandão M, Poggio F, Addeo A, Lambertini M. VISTA: A Promising Target for Cancer Immunotherapy? Immunotargets Ther. 2021 Jun 22;10:185-200. doi: 10.2147/ITT.S260429. PMID: 34189130; PMCID: PMC8235942.
- Huang X, Zhang X, Li E, Zhang G, Wang X, Tang T, Bai X, Liang T. VISTA: an immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J Hematol Oncol. 2020 Jun 29;13(1):83. doi: 10.1186/s13045-020-00917-y. PMID: 32600443; PMCID: PMC7325042.
- Yuan L, Tatineni J, Mahoney KM, Freeman GJ. VISTA: A Mediator of Quiescence and a Promising Target in Cancer Immunotherapy. Trends Immunol. 2021 Mar;42(3):209-227. doi: 10.1016/j.it.2020.12.008. Epub 2021 Jan 23. PMID: 33495077; PMCID: PMC8088836.
- Mehdizadeh S, Bayatipoor H, Pashangzadeh S, Jafarpour R, Shojaei Z, Motallebnezhad M. Immune checkpoints and cancer development: Therapeutic implications and future directions. Pathol Res Pract. 2021 Jul;223:153485. doi: 10.1016/j.prp.2021.153485. Epub 2021 May 15. PMID: 34022684.
- Liu B, Cheng L, Gao H, Zhang J, Dong Y, Gao W, Yuan S, Gong T, Huang W. The biology of VSIG4: Implications for the treatment of immune-mediated inflammatory diseases and cancer. Cancer Lett. 2023 Jan 28;553:215996. doi: 10.1016/j.canlet.2022.215996. Epub 2022 Nov 5. PMID: 36343787.
- Widyagarini A, Nishii N, Kawano Y, Zhang C, Azuma M. VSIG4/CRIg directly regulates early CD8+ T cell activation through its counter-receptor in a narrow window. Biochem Biophys Res Commun. 2022 Jul 23;614:100-106. doi: 10.1016/j.bbrc.2022.04.120. Epub 2022 Apr 29. PMID: 35576680.
- Yang S, Wei W, Zhao Q. B7-H3, a checkpoint molecule, as a target for cancer immunotherapy. Int J Biol Sci. 2020 Mar 25;16(11):1767-1773. doi: 10.7150/ijbs.41105. PMID: 32398947; PMCID: PMC7211166.
- Zhao B, Li H, Xia Y, Wang Y, Wang Y, Shi Y, Xing H, Qu T, Wang Y, Ma W. Immune checkpoint of B7-H3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol. 2022 Oct 25;15(1):153. doi: 10.1186/s13045-022-01364-7. PMID: 36284349; PMCID: PMC9597993.
- Wennerberg E, Mukherjee S, Sainz RM, Stiles BM. The ART of tumor immune escape. Oncoimmunology. 2022 May 14;11(1):2076310. doi: 10.1080/2162402X.2022.2076310. PMID: 35602287; PMCID: PMC9116389.
- Freed-Pastor WA, Lambert LJ, Ely ZA, Pattada NB, Bhutkar A, Eng G, Mercer KL, Garcia AP, Lin L, Rideout WM 3rd, Hwang WL, Schenkel JM, Jaeger AM, Bronson RT, Westcott PMK, Hether TD, Divakar P, Reeves JW, Deshpande V, Delorey T, Phillips D, Yilmaz OH, Regev A, Jacks T. The CD155/TIGIT axis promotes and maintains immune evasion in neoantigen-expressing pancreatic cancer. Cancer Cell. 2021 Oct 11;39(10):1342-1360.e14. doi: 10.1016/j.ccell.2021.07.007. Epub 2021 Aug 5. PMID: 34358448; PMCID: PMC8511341.
- Nandi SS, Gohil T, Sawant SA, Lambe UP, Ghosh S, Jana S. CD155: A Key Receptor Playing Diversified Roles. Curr Mol Med. 2022;22(7):594-607. doi: 10.2174/1566524021666210910112906. PMID: 34514998.
- Jorgensen MM, de la Puente P. Leukemia Inhibitory Factor: An Important Cytokine in Pathologies and Cancer. Biomolecules. 2022 Jan 27;12(2):217. doi: 10.3390/biom12020217. PMID: 35204717; PMCID: PMC8961628.
- Zhou X, Du J, Liu C, Zeng H, Chen Y, Liu L, Wu D. A Pan-Cancer Analysis of CD161, a Potential New Immune Checkpoint. Front Immunol. 2021 Jul 9;12:688215. doi: 10.3389/fimmu.2021.688215. PMID: 34305920; PMCID: PMC8299557.
- Lin J, Guo D, Liu H, Zhou W, Wang C, Müller I, Kossenkov AV, Drapkin R, Bitler BG, Helin K, Zhang R. The SETDB1-TRIM28 Complex Suppresses Antitumor Immunity. Cancer Immunol Res. 2021 Dec;9(12):1413-1424. doi: 10.1158/2326-6066.CIR-21-0754. PMID: 34848497; PMCID: PMC8647838.




