Does ANI-7 bring good news to breast cancer patients?
Abstract
ANI-7 is a compound that activates the AhR pathway and inhibits the growth of various cancer cells, including MCF-7 breast cancer cells, with a GI50 of 0.56 μM. It also shows inhibitory effects on MCF10A and MDA-MB-468 cell lines. The activation of the AhR pathway by ANI-7 induces CYP1 metabolic monooxygenase, DNA damage, and checkpoint kinase activation, leading to cell cycle arrest in the S phase and cell death in sensitive breast cancer cell lines. ANI-7 is a promising tool for studying the AhR pathway in breast cancer.
Introduction
Despite breakthrough advances in cancer treatment, more than half a million women worldwide still die from breast cancer each year. Additionally, no targeted therapies for triple-negative breast cancers currently lack estrogen, progesterone, and HER-2 hormone receptors. Moreover, resistance to common broad-spectrum chemotherapeutic drugs is an urgent problem that needs to be addressed. There is an urgent need for new drugs and means to address this deficiency.
In the past few years, several research teams have discovered that developing new drugs mediated by the aryl hydrocarbon receptor (AhR) pathway may be a good choice as a promising treatment method for breast cancer. While the AhR pathway is traditionally known for metabolizing environmental toxins, it also plays a role in initiating and maintaining breast cancer growth.
Key oncogenic roles of AhR include immunosuppression, tumorigenesis, regulation of genes involved in energy and xenobiotic metabolism, and subsequent roles in cell proliferation and the cell cycle. Upregulation of AhR in the cytoplasm of inflammatory breast cancer cells correlates with poor prognosis, making AhR an attractive target for breast cancer therapy.
What is ANI-7
ANI-7 is an activator of the AhR pathway. ANI-7 inhibits the growth of a variety of cancer cells and selectively inhibits the growth of MCF-7 breast cancer cells with a GI50 of 0.56 μM. Moreover, ANI-7 also has significant inhibitory effects on MCF10A and MDA-MB-468 cell lines. ANI-7 induces CYP1 metabolic monooxygenase by activating the AhR pathway, inducing DNA damage, checkpoint kinase (Chk2) activation, cell cycle arrest in the S phase, and cell death in sensitive breast cancer cell lines. Overall, ANI-7 is a promising tool for studying the AhR pathway in breast cancer.
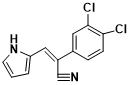
Structure of ANI-7
The research process of ANI-7 inhibits the growth of various cancer cells.
Immunotherapy inhibits tumor development by activating the body’s immune system. Despite the breakthrough success of this new class of therapies, currently available drugs benefit only a minority of patients. The scientists hope that further research into the molecular mechanisms of how the immune system is suppressed will provide important information for the development of new immunotherapies.
The aryl hydrocarbon receptor, or AHR for short, is also known as the dioxin receptor because it mediates the toxic effects of dioxins. But in addition to external toxins, AHR is also activated by the body’s metabolites, such as tryptophan metabolites. Tryptophan is an essential amino acid that the body obtains through food. Past research has found that degradation products produced by tryptophan metabolism promote the mobility of cancer cells and weaken the immune system’s anti-tumor response. Therefore, targeting the tryptophan metabolizing enzyme-AHR pathway has become a strategy for the development of immunotherapy in recent years.
The study of ANI-7’s involvement in inhibiting the growth of various cancer cells involves multiple steps. ANI-7 is a small molecule inhibitor of AHR developed through medicinal chemistry optimization. The researchers examined the effect of ANI-7 on the growth and survival of a variety of cancer cells, including breast, lung, and colon cancer cells, and they found that ANI-7 induces CYP1 metabolic monooxygenase by activating the AhR pathway, which is in sensitive breast cancer cells Induces DNA damage, checkpoint kinase (Chk2) activation, cell cycle arrest in S phase, and cell death in the lineage. The researchers tested the efficacy of ANI-7 in animal models of cancer. They found that ANI-7 inhibited tumor growth and prolonged the survival of mice implanted with cancer cells.
The research process of ANI-7 inhibiting the growth of various cancer cells involves many aspects such as drug development, in vitro and in vivo research, mechanism research, and clinical trials. Therefore, the latest progress needs further research.
The mechanism of action of ANI-7 on MCF-7 breast cancer
Breast cancer is a significant health concern, and despite advances in treatment, it remains a leading cause of cancer-related deaths worldwide. ANI-7 is a novel compound that has demonstrated promising anticancer activity against MCF-7 breast cancer cells. The mechanism of action of ANI-7 on MCF-7 breast cancer involves multiple pathways that lead to cancer cell death.
One of the key mechanisms of ANI-7’s action is its ability to induce apoptosis, a programmed cell death process crucial for removing damaged or abnormal cells. ANI-7 promotes apoptosis by activating caspases, a family of proteases that are essential for the execution of apoptosis. ANI-7 also increases the expression of pro-apoptotic genes such as Bax and decreases the expression of anti-apoptotic genes such as Bcl-2, resulting in a shift towards a pro-apoptotic state.
ANI-7 also inhibits the activity of the PI3K/Akt/mTOR pathway, which is a signaling pathway that is frequently deregulated in cancer cells. This pathway plays a critical role in cell growth, survival, and proliferation. ANI-7 inhibits the activity of PI3K, preventing the activation of Akt and mTOR. This leads to a decrease in the levels of cyclin D1, a protein that is essential for cell cycle progression, and an increase in the levels of p27, a cyclin-dependent kinase inhibitor that inhibits cell cycle progression.
Another mechanism of ANI-7’s action is its ability to disrupt the microtubule network, which is essential for cell division. ANI-7 binds to tubulin, a protein that forms the building blocks of microtubules, and inhibits its polymerization, disrupting the microtubule network. This disrupts cell division and leads to the accumulation of cells in the G2/M phase of the cell cycle, ultimately resulting in cell death.
In conclusion, ANI-7 demonstrates significant anticancer activity against MCF-7 breast cancer cells through multiple mechanisms of action, including the induction of apoptosis, inhibition of the PI3K/Akt/mTOR pathway, and disruption of the microtubule network. These findings suggest that ANI-7 has significant potential as a novel therapeutic agent for breast cancer. However, further research is needed to fully understand its clinical potential and to determine its safety and efficacy in human trials.
Summary
Breast cancer is a phenomenon of uncontrolled proliferation of breast epithelial cells under the action of various carcinogenic factors. In the early stage of the disease, it is often manifested as breast lumps, nipple discharge, axillary lymph node enlargement, and other symptoms. In the late stage, distant metastasis can occur due to cancer cells, and multiple organ lesions appear, which directly threaten the life of patients. Breast cancer is often called ‘ pink killer ‘, and its incidence ranks first in female malignant tumors. Male breast cancer is rare. With the improvement of medical levels, breast cancer has become one of the most effective solid tumors. So far, no drugs for breast cancer have been marketed. Therefore, the task of drug research for breast cancer is imminent.
As an effective selective breast cancer cell inhibitor, ANI-7 has a GI50 of 0.5 μM for MCF-7. ANI-7 induces CYP1 metabolic monooxygenase by activating the aromatic hydrocarbon receptor ( AHR ) pathway and induces DNA damage, checkpoint activation ( CHK2 ), S-phase cell cycle arrest, and cell death in sensitive breast cancer cell lines. It is believed that shortly, with the unremitting efforts of researchers, ANI-7 or other drugs for breast cancer will eventually be on the market, bringing hope to breast cancer patients.
References
- Gilbert, J., De Iuliis, G. N., Tarleton, M., McCluskey, A., & Sakoff, J. A. (2018). (Z)-2-(3, 4-Dichlorophenyl)-3-(1H-pyrrol-2-yl) acrylonitrile exhibits selective antitumor activity in breast cancer cell lines via the aryl hydrocarbon receptor pathway. Molecular pharmacology, 93(2), 168-177.
- Baker, J. R., Gilbert, J., Paula, S., Zhu, X., Sakoff, J. A., & McCluskey, A. (2018). Dichlorophenylacrylonitriles as AhR ligands that display selective breast cancer cytotoxicity in vitro. ChemMedChem, 13(14), 1447-1458.
- Tarleton, M., Gilbert, J., Robertson, M. J., McCluskey, A., & Sakoff, J. A. (2011). Library synthesis and cytotoxicity of a family of 2-phenylacrylonitriles and discovery of an estrogen-dependent breast cancer lead compound. MedChemComm, 2(1), 31-37.
- Sharma, P., & Allison, J. P. (2015). The future of immune checkpoint therapy. Science, 348(6230), 56-61.
- Opitz, C. A., Litzenburger, U. M., Sahm, F., Ott, M., Tritschler, I., Trump, S., … & Platten, M. (2011). An endogenous tumor-promoting ligand of the human aryl hydrocarbon receptor. Nature, 478(7368), 197-203.