Economic Considerations and Future Outlook for Resmetirom in the Treatment of MASH
Abstract
The rising global prevalence of metabolic dysfunction-associated steatohepatitis (MASH) has highlighted the need for effective and economically viable treatments. Resmetirom, the first FDA-approved drug for MASH, offers a promising therapeutic option with its ability to reduce liver fat, improve liver function, and slow disease progression. This article discusses the economic considerations surrounding resmetirom’s potential cost-effectiveness, which is projected to meet widely accepted thresholds if priced appropriately. However, the long-term benefits, particularly in reducing severe complications such as cirrhosis and liver cancer, remain to be fully established. Additionally, the potential for combination therapies involving resmetirom and other treatments for comorbid conditions, such as obesity and diabetes, adds complexity to its economic evaluation. As clinical trials continue and more data on resmetirom’s long-term efficacy becomes available, the future outlook for MASH treatment appears promising, with the potential for combination approaches to improve patient outcomes and reduce healthcare costs.
Introduction to MASH and Its Growing Prevalence
Metabolic dysfunction-associated steatohepatitis (MASH) has emerged as a critical health concern, affecting millions globally. Once known as non-alcoholic steatohepatitis (NASH), this liver condition is now recognized as a part of the broader spectrum of metabolic dysfunction-associated liver diseases (MASLD), highlighting its association with metabolic syndrome. MASH is characterized by liver inflammation, fat accumulation, and, in some cases, fibrosis. It is considered a more advanced stage of non-alcoholic fatty liver disease (NAFLD), and its progression can lead to severe liver damage, including cirrhosis and liver failure.
The rising global incidence of MASH is closely linked to the increasing prevalence of obesity, Type 2 diabetes, and cardiovascular diseases—conditions that are integral to metabolic syndrome. Estimates suggest that one-third of the world’s population is affected by some form of fatty liver disease, with a significant portion progressing to MASH, especially in individuals with obesity or diabetes. The condition is often underdiagnosed because its symptoms are subtle and can be mistaken for other diseases, leading to a delay in treatment.
MASH has gained attention as a major cause of liver-related morbidity and mortality. According to recent studies, individuals with MASH face a heightened risk of cardiovascular events, liver cirrhosis, and even liver cancer. It is crucial to address this condition early to prevent progression to more severe stages. Unfortunately, the diagnostic methods available, such as liver biopsy, remain invasive and often impractical for large-scale screening.
As the understanding of MASH evolves, there is a pressing need for innovative therapies and diagnostic tools to better manage the disease. The FDA’s approval of the first drug for MASH, resmetirom, represents a significant step forward in addressing this unmet medical need. However, further research is essential to improve early detection, treatment efficacy, and long-term patient outcomes.
Resmetirom: A Breakthrough in MASH Treatment
Resmetirom represents a groundbreaking advancement in the treatment of metabolic dysfunction-associated steatohepatitis (MASH). As the first FDA-approved medication specifically targeting this condition, resmetirom offers hope for individuals suffering from MASH, a severe stage of non-alcoholic fatty liver disease (NAFLD) that can lead to liver fibrosis, cirrhosis, and even liver cancer. MASH is increasingly recognized as a major global health issue, largely due to its close association with metabolic conditions such as obesity, Type 2 diabetes, and cardiovascular disease.
The mechanism of action of resmetirom is centered on its ability to selectively activate the thyroid hormone receptor beta (THR-β) in the liver. Unlike other thyroid hormone receptor agonists that can affect multiple organs, resmetirom specifically targets the liver. By stimulating THR-β, resmetirom enhances liver fat metabolism, improves mitochondrial function, and reduces lipotoxicity—processes that are critical for managing the fatty liver component of MASH. This liver-specific action minimizes the risk of unwanted side effects that are typically seen with other thyroid hormone treatments, which affect organs like the heart and bones.
Clinical trials have shown that resmetirom significantly reduces liver fat content and improves liver function in patients with MASH. The MAESTRO-NAFLD and MAESTRO-NASH trials provided compelling evidence of its efficacy, with resmetirom showing improvements in liver fat, cholesterol levels, and liver stiffness. Importantly, resmetirom also improved patients’ health-related quality of life, making it not only a promising treatment for liver health but also for overall well-being.
Despite its promising results, resmetirom’s journey is not without challenges. As with any new treatment, its long-term safety and potential for wider use in combination with other therapies (e.g., GLP-1 agonists for obesity and diabetes) will need to be closely monitored. However, resmetirom’s FDA approval marks a historic moment in the fight against MASH, offering a targeted, effective, and relatively safe option for patients battling this complex disease.
Clinical Trials and Efficacy
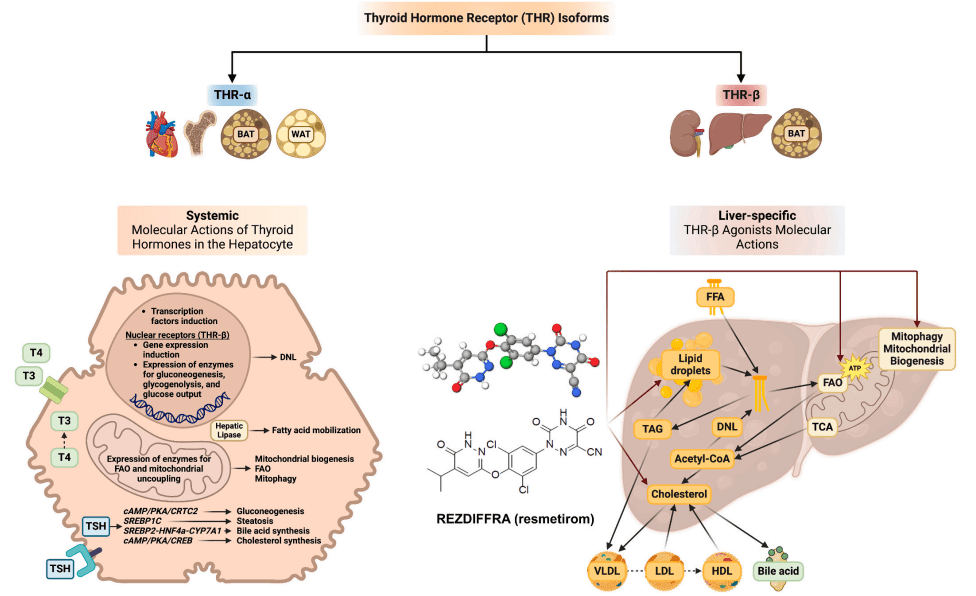
Fig. 1. Molecular actions of thyroid hormones in the hepatocyte and liver-specific molecular actions of resmetirom.
Resmetirom has undergone rigorous clinical testing to establish its safety and efficacy in treating metabolic dysfunction-associated steatohepatitis (MASH). The most notable studies are the MAESTRO-NAFLD and MAESTRO-NASH trials, which provided significant evidence supporting its use as an effective treatment option. These studies were pivotal in demonstrating the drug’s ability to improve liver fat content, reduce liver stiffness, and alleviate other markers of liver dysfunction, providing a much-needed therapeutic approach for a condition that has historically lacked targeted treatments.
The MAESTRO-NAFLD1 trial, a Phase 3 randomized, double-blind study, explored the impact of resmetirom on patients with non-alcoholic fatty liver disease (NAFLD) and varying degrees of liver fibrosis. Results showed a marked reduction in liver fat, particularly in patients receiving the higher doses of resmetirom. Additionally, resmetirom demonstrated a favorable impact on lipid metabolism, with significant reductions in LDL cholesterol and triglycerides, which are crucial in managing cardiovascular risk—a common co-morbidity in patients with MASH.
The MAESTRO-NASH trial expanded on these findings, focusing on patients with NASH and liver fibrosis. Resmetirom was shown to promote the resolution of NASH without worsening fibrosis, an important breakthrough, as this specific improvement is rare with current therapies. Patients treated with resmetirom experienced a significant reduction in hepatic fat content and marked improvements in liver stiffness, suggesting that the drug could slow the progression of fibrosis.
Despite the encouraging results, clinical trials have also highlighted the need for ongoing monitoring. The most common side effects noted in the trials were mild gastrointestinal symptoms, such as diarrhea and nausea, which were transient and typically occurred during the initial stages of treatment. Importantly, no severe safety concerns were raised, and long-term data from ongoing studies will provide further insights into resmetirom’s safety profile and its potential for combination therapies.
The clinical data thus far strongly support resmetirom as a promising treatment for MASH, offering patients a new and targeted therapeutic option for managing this complex disease.
Challenges in Diagnosis and Treatment
Despite the promising advancements in treatment for metabolic dysfunction-associated steatohepatitis (MASH), challenges in both diagnosis and treatment persist, creating significant barriers to effective management of the disease. One of the primary difficulties in diagnosing MASH is its often subtle presentation and the overlap of symptoms with other metabolic conditions, such as obesity and Type 2 diabetes. Unlike some diseases, MASH does not always present with overt symptoms until it has progressed to advanced stages, such as liver fibrosis or cirrhosis. This often leads to delayed diagnosis, which can hinder timely intervention and treatment.
Traditional diagnostic methods, such as liver biopsy, while considered the gold standard, come with limitations. Liver biopsy is invasive, costly, and carries some risks, including bleeding and infection. Furthermore, it is not a practical option for large-scale screening, especially in populations at high risk for developing MASH. As a result, there has been growing interest in non-invasive diagnostic tools, such as elastography and biomarkers, to assess liver stiffness and fat content. However, while these tools show promise, they are not yet universally reliable or widely available for routine use.
In terms of treatment, while drugs like resmetirom have shown effectiveness in clinical trials, a major challenge remains in addressing the multifaceted nature of MASH. The condition often coexists with other metabolic disorders, including obesity, insulin resistance, and cardiovascular disease, requiring a holistic treatment approach. Combination therapies targeting different aspects of the disease—such as resmetirom alongside glucagon-like peptide-1 (GLP-1) receptor agonists for obesity or diabetes—are a promising area of research but have yet to be fully realized in clinical practice.
Moreover, while resmetirom has shown substantial promise, its long-term safety and efficacy in broader populations remain under investigation. It will be critical to monitor the effects of such treatments over extended periods, particularly considering potential risks such as drug interactions or side effects.
As more research is conducted, improved diagnostic tools and treatment combinations will hopefully emerge, allowing for more personalized and effective management of MASH.
Economic Considerations and Future Outlook
As the prevalence of metabolic dysfunction-associated steatohepatitis (MASH) continues to rise globally, understanding the economic impact of treatment options is becoming increasingly important. The introduction of resmetirom, the first FDA-approved drug for MASH, marks a significant step forward in the management of this complex condition. However, as with any new medication, the cost-effectiveness of resmetirom must be carefully evaluated in light of its potential benefits for patients and the healthcare system.
Initial studies suggest that resmetirom may meet commonly accepted cost-effectiveness thresholds if priced appropriately. The Institute for Clinical and Economic Review (ICER) has estimated that resmetirom could be cost-effective at a price range of $39,600 to $50,100 annually, assuming that improvements in liver fibrosis and reductions in cirrhosis-related healthcare costs are realized over time. Such calculations are based on the premise that resmetirom’s ability to slow the progression of liver disease would result in long-term savings by reducing the need for more expensive treatments like liver transplants or hospitalization for complications of cirrhosis.
However, there are challenges to this cost-effectiveness analysis. For one, the true long-term impact of resmetirom on reducing complications like cirrhosis and liver cancer is still under investigation. Clinical trials have shown promising results in terms of improving liver fat content and fibrosis, but longer follow-up studies are needed to confirm whether these improvements translate into significant reductions in liver-related morbidity and mortality. Additionally, the potential for resmetirom to be used in combination with other therapies, such as glucagon-like peptide-1 (GLP-1) agonists for obesity and Type 2 diabetes, could complicate cost assessments and require more nuanced economic evaluations.
Looking ahead, the future of MASH treatment appears promising, with ongoing clinical trials and research focused on improving both the safety and effectiveness of resmetirom and other potential therapies. It is likely that combination therapies and more targeted treatment regimens will emerge as the preferred approach for managing MASH, offering patients a more comprehensive solution to this multifaceted disease.