Enhancing Topoisomerase I Inhibitor-Based Antibody-Drug Conjugates: Strategies and Future Directions in Cancer Therapy
Abstract
Topoisomerase I inhibitor-based antibody-drug conjugates (TOP1-ADCs) represent a promising class of targeted cancer therapies that combine the potency of topoisomerase inhibitors with the precision of antibody-based delivery. These conjugates aim to enhance the therapeutic efficacy of cancer treatment by selectively targeting tumor cells, reducing systemic toxicity, and overcoming some of the limitations of traditional chemotherapy. This article explores the development of TOP1-ADCs, highlighting their design considerations, including antibody selection, linker technology, and drug payloads. It also compares their therapeutic potential to conventional anti-cancer drugs and discusses challenges such as drug resistance and optimizing the drug-to-antibody ratio. Furthermore, the paper delves into future directions, including combinatorial strategies with immunotherapy and chemotherapy, to maximize the clinical benefits of TOP1-ADCs. As research in this area continues, TOP1-ADCs hold the promise of revolutionizing cancer treatment with more effective, targeted therapies.
Introduction
The DNA topoisomerase enzymes are pivotal for cell function and are found ubiquitously in all domains of life. The various topoisomerase enzymes have roles in a wide range of functions related to the maintenance of DNA topology during DNA replication and transcription. Topoisomerases support the fidelity of DNA replication and transcription by preventing or correcting topological problems, such as torsion, that may arise in double-helical DNA during its biochemical manipulation in the process of DNA replication or transcription.
Topoisomerase cleavage complexes (TOPcc), in which topoisomerases are bound to DNA breaks, are integral to topoisomerase-mediated changes in DNA topology but, importantly, also pose potential threats to genomic integrity. For example, trappingof a TOPcc in advance of the replication machinery or during chromosome segregation, where interwoven DNA helices are unlinked by topoisomerases, can have adverse effects on genomic stability and, hence, cell viability . Topoisomerase inhibitors act by stabilizing DNA–topoisomerase complexes, leading to double-strand breaks. Among the six human topoisomerases (TOP1, TOP1MT, TOP2a, TOP2b, TOP3a, and TOP3b), TOP1 is essential to genomic stability due to its removal of both positive and negative DNA supercoils that might otherwise lead to DNA breaks.
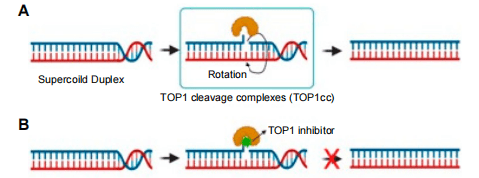
Figure 1. TOP1 and TOP1 inhibitor mechanism. (A) TOP1 forms 3′-phosphotyrosine bonds, allowing the cut DNA strand to rotate about the intact one, thus relaxing DNA supercoiling. (B) A TOP1 inhibitor binds at the TOP1 enzyme–DNA interface to prevent DNA re-ligation and to lock the enzyme into TOP1cc.
Despite their success, camptothecin derivatives have limitations, including poor solubility and severe side effects. As a result, ongoing research has focused on developing more effective and targeted therapies. One promising strategy involves the use of antibody-drug conjugates (ADCs), which combine the specificity of monoclonal antibodies with the potent cytotoxicity of topoisomerase inhibitors. By conjugating these inhibitors to antibodies targeting tumor-specific antigens, researchers aim to improve the selectivity and accumulation of the drug in cancer cells, reducing off-target effects and enhancing therapeutic efficacy.
Developing Topoisomerase I Inhibitor-Based Antibody-Drug Conjugates (TOP1-ADCs)
Antibody-drug conjugates (ADCs) represent a promising strategy for targeted cancer therapy. These biologic agents are designed by linking a potent cytotoxic drug to a monoclonal antibody that selectively targets tumor-specific antigens, allowing for precise drug delivery to cancer cells while minimizing damage to healthy tissues. The concept behind ADCs is to exploit the targeting ability of antibodies to direct highly potent drugs directly to tumor sites, overcoming the limitations of traditional chemotherapy, which affects both cancerous and normal cells indiscriminately.
The development of topoisomerase I (TOP1) inhibitor-based ADCs is a groundbreaking approach to improving cancer treatment. TOP1 inhibitors, such as camptothecin derivatives, work by stabilizing the transient cleavage complexes formed by the interaction of TOP1 with DNA, preventing the religation of the DNA strand and inducing cell death. However, due to their poor solubility and side effects, the efficacy of these inhibitors can be limited. By conjugating TOP1 inhibitors to monoclonal antibodies, researchers can deliver these drugs more specifically to tumor cells that overexpress certain antigens, such as HER2 or CD22, which are commonly found in breast cancer and leukemia, respectively.
One of the key advantages of TOP1-ADCs is their ability to concentrate the therapeutic payload directly within cancer cells. For example, T-DM1, an ADC that links the HER2-targeting antibody trastuzumab to the cytotoxic drug DM1 (a derivative of maytansine), has shown significant clinical success in treating HER2-positive breast cancer. Similarly, other TOP1-ADC candidates are under investigation, demonstrating enhanced therapeutic outcomes compared to traditional chemotherapy agents by delivering the potent TOP1 inhibitors specifically to malignant cells.
The development of TOP1-ADCs also involves optimizing several factors, including the selection of suitable antibodies, linker technology, and drug payloads to maximize both efficacy and safety. In the next section, we will explore how these ADCs compare to traditional anti-cancer therapies in terms of therapeutic potential.
Enhanced Efficacy of TOP1-ADCs vs. Traditional Anti-Cancer Drugs
Topoisomerase I inhibitor-based antibody-drug conjugates (TOP1-ADCs) have shown significant promise in enhancing the therapeutic efficacy of cancer treatment compared to traditional anti-cancer drugs. Traditional chemotherapy drugs, although effective, often suffer from limitations such as systemic toxicity, poor selectivity, and resistance development. These drugs affect both cancerous and normal cells, leading to severe side effects such as nausea, immune suppression, and hair loss. In contrast, TOP1-ADCs aim to target tumor cells specifically, reducing off-target effects and improving the overall safety profile.
One of the key advantages of TOP1-ADCs is their ability to deliver highly potent cytotoxic agents directly to cancer cells. The antibody component of the ADC is designed to bind selectively to tumor-specific antigens, facilitating the precise delivery of the topoisomerase inhibitor to the tumor site. This targeted delivery not only enhances the concentration of the drug within the tumor but also minimizes exposure to healthy tissues, thus reducing systemic toxicity.
For example, T-DM1, an ADC that combines the HER2-targeting antibody trastuzumab with the cytotoxic drug DM1 (a derivative of maytansine), has demonstrated superior efficacy in treating HER2-positive breast cancer compared to conventional chemotherapy. Clinical trials have shown that T-DM1 significantly improves progression-free survival and overall response rates in patients with metastatic HER2-positive breast cancer, making it a promising alternative to traditional treatments. Similarly, other TOP1-ADC candidates targeting different tumor-associated antigens are being evaluated in clinical trials, showing encouraging results.
In addition to their enhanced efficacy, TOP1-ADCs offer the potential for overcoming drug resistance, a common challenge in cancer treatment. By specifically targeting tumor cells and utilizing the precise delivery of the cytotoxic payload, these drugs can bypass some of the mechanisms that cancer cells use to evade traditional therapies, such as drug efflux pumps and mutations in drug targets.
Overall, the development of TOP1-ADCs represents a significant advancement in cancer treatment, offering higher therapeutic potential with fewer side effects compared to conventional anti-cancer drugs.
Designing Effective TOP1-ADCs: Key Considerations and Challenges
The design and construction of topoisomerase I inhibitor-based antibody-drug conjugates (TOP1-ADCs) involve several crucial factors that impact both their efficacy and safety profile. These include the selection of an appropriate monoclonal antibody, the choice of the drug payload, the design of the linker technology, and the optimization of the conjugation process. Each of these elements must be carefully considered to ensure that the final ADC is both effective in targeting cancer cells and capable of delivering a potent cytotoxic dose without causing excessive toxicity to healthy tissues.
- Antibody Selection: The monoclonal antibody used in the ADC must specifically recognize a tumor-associated antigen that is overexpressed on the surface of cancer cells. This selectivity ensures that the drug is delivered to the tumor site, minimizing off-target effects. For example, in HER2-positive breast cancer, trastuzumab is commonly used as the antibody component, as it targets the HER2 receptor, which is overexpressed on the surface of tumor cells.
- Linker Technology: The linker is a key component in ADC design, as it connects the antibody to the drug payload. The linker must be stable in the bloodstream to prevent premature release of the toxic drug but also be designed to release the drug efficiently once the ADC reaches the tumor. The stability and cleavage rate of the linker are critical factors in determining the success of the ADC.
- Drug Payload Selection: The cytotoxic drug payload must be potent enough to kill cancer cells at low concentrations but should not cause severe systemic toxicity. For TOP1-ADCs, camptothecin derivatives, such as SN-38 or DM1, are commonly used as payloads because of their ability to inhibit topoisomerase I and induce DNA damage in rapidly dividing tumor cells.
Despite these advancements, several challenges remain in the design of TOP1-ADCs. Issues such as optimizing the drug-to-antibody ratio, overcoming drug resistance mechanisms, and managing immunogenicity are ongoing areas of research. Continued improvements in ADC design will be crucial to their success in clinical settings.
Future Directions and Strategies to Maximize TOP1-ADCs’ Potential
TOP1 inhibitors have shown considerable potential as therapeutic agents against cancers; however, their clinical application has been hindered by their adverse pharmacokinetic profiles and off-target toxicities. Both obstacles can be mitigated by targeting their delivery via tumor-specific antibodies, as in TOP1-ADC formulations. The strategically chosen antibody must specifically and selectively target an antigen expressed on tumor cells; by using different tumor-targeting antibodies, the spectrum of tumor types is broadened. The crosslinker design should not only confer stability to the conjugate, minimizing premature drug release, but also allow for efficient release in the cancer cell. Additionally, conjugation of the linker to the antibody must not compromise its specificity nor should linker–drug conjugation degrade the desirable chemical properties of the payload. In turn, the cytotoxic payload, a TOP1 inhibitor, should be formulated to optimize both cytotoxicity and conjugation site(s). A final consideration in TOP1-ADC formulation is that higher drug-to-antibody ratios (DARs) may further improve their therapeutic efficacy. Further research on various anticancer drugs and cancer cell targets is needed to identify additional candidates for combination with TOP1 inhibitors. The development of multi-drug ADCs consisting of TOP1 and a second anticancer agent have considerable potential for synergistic anticancer effects, especially in tumors that have developed resistance to primary therapies.
- Combinatorial Therapies with Immunotherapy: One of the most exciting developments in cancer treatment is the combination of ADCs with immunotherapy. Immunotherapies, such as immune checkpoint inhibitors, have revolutionized the treatment of several cancers by boosting the body’s immune response to tumor cells. Combining TOP1-ADCs with checkpoint inhibitors like PD-1/PD-L1 antibodies could enhance the immune system’s ability to recognize and destroy cancer cells. This approach could help overcome the immunosuppressive environment of many tumors and potentially improve the efficacy of ADCs.
- Co-Administration with Chemotherapy: Another promising strategy involves the co-administration of TOP1-ADCs with traditional chemotherapy agents. By combining the precise targeting of ADCs with the broad tumor-killing effects of chemotherapy, researchers hope to enhance the overall anti-cancer effect. For example, combining a TOP1-ADC with a chemotherapy drug that targets a different pathway could increase the chances of inducing complete tumor regression. This strategy might be particularly useful in cases where resistance to either therapy occurs.
- Targeting Drug Resistance Mechanisms: Drug resistance remains one of the most significant challenges in cancer therapy. Many tumors develop resistance to traditional chemotherapies and targeted therapies by utilizing mechanisms such as drug efflux pumps, mutations in drug targets, and evasion of apoptosis. Research is underway to develop ADCs that can bypass these resistance mechanisms by using novel linkers, drug payloads, and antibodies that target alternative antigens. Additionally, combining ADCs with inhibitors of resistance pathways is a promising avenue for overcoming this barrier.
In conclusion, while TOP1-ADCs have shown significant promise as cancer therapeutics, ongoing research and the exploration of combinatorial strategies will be crucial for maximizing their full therapeutic potential. The future of cancer treatment lies in personalized, multi-pronged approaches that harness the full power of targeted and immune-based therapies.
References
- Han, S.; Lim, K.S.; Blackburn, B.J.; Yun, J.; Putnam, C.W.; Bull, D.A.; Won, Y.-W. (2022). The Potential of Topoisomerase Inhibitor-Based Antibody–Drug Conjugates. Pharmaceutics, 14, 1707.
- Pommier, Y., Leo, E., Zhang, H., & Marchand, C. (2010). DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chemistry & Biology, 17(5), 421-433.
- O’Connor, M. J. (2015). Targeting the DNA damage response in cancer. Molecular Cell, 60(4), 547-560.
- Shi, Y., & Dolginow, S. (2001). Development of topoisomerase I inhibitors for cancer therapy. Anti-Cancer Drugs, 12(4), 305-315.
- DeAngelis, S. (2019). Antibody-drug conjugates in the treatment of cancer. Current Opinion in Oncology, 31(5), 440-446.
- Swenson, L., & Mowry, L. (2017). Topoisomerase inhibitors: Antibody-drug conjugates for targeted cancer therapy. Journal of Clinical Oncology, 35(23), 2636-2645.
- Nunes, M. E., & Cummings, M. D. (2016). Designing topoisomerase I-based antibody-drug conjugates. Future Medicinal Chemistry, 8(9), 1109-1123.
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48.
- Lambert, J. M., & Morris, C. Q. (2017). Antibody-drug conjugates for the treatment of cancer. Nature Reviews Clinical Oncology, 14(5), 223-241.
- Chari, R. V. (2008). Targeted therapy with antibody-drug conjugates. Molecular Cancer Research, 6(5), 1489-1497.
- Verma, S., & Miles, D. (2012). Trastuzumab emtansine for HER2-positive breast cancer. The New England Journal of Medicine, 367(19), 1783-1791.
- Senter, P. D. (2013). The development of antibody-drug conjugates: A history and preview. Cancer Research, 73(8), 3307-3315.
- Yu, M., & Zhang, S. (2016). Antibody-drug conjugates: From discovery to clinical development. Nature Reviews Drug Discovery, 15(1), 97-110.
- Porell, E. M., & Miller, G. T. (2015). Linker technologies in the development of antibody-drug conjugates. Future Medicinal Chemistry, 7(8), 1061-1078.




