Everything You Need to Know About Ensartinib for ALK-Positive Non-Small Cell Lung Cancer
Abstract
Ensartinib is a targeted therapy that has revolutionized the treatment of ALK-positive non-small cell lung cancer (NSCLC). Approved by the FDA in December 2024, Ensartinib works by inhibiting the ALK protein, which plays a key role in the development of cancer in patients with specific genetic mutations. This blog post explores Ensartinib’s mechanism of action, clinical efficacy, and safety profile based on pivotal clinical trials, particularly the eXALT3 trial, which demonstrated its superior performance compared to other ALK inhibitors. It also discusses the potential benefits of Ensartinib for treating brain metastases, a common challenge in ALK-positive NSCLC. The post concludes by emphasizing the broader implications of Ensartinib for the future of targeted therapies in oncology and the importance of personalized treatment plans for patients with ALK-positive lung cancer.
Lung cancer remains one of the most significant global health challenges, claiming millions of lives each year. Among its various types, non-small cell lung cancer (NSCLC) is the most common, accounting for approximately 85% of all cases. However, ALK-positive NSCLC, a specific subtype driven by a genetic mutation in the anaplastic lymphoma kinase (ALK) gene, has seen a game-changing shift in treatment options thanks to targeted therapies like Ensartinib. This blog will delve into Ensartinib’s role in treating ALK-positive lung cancer, its effectiveness, side effects, and what the future holds for this promising drug.
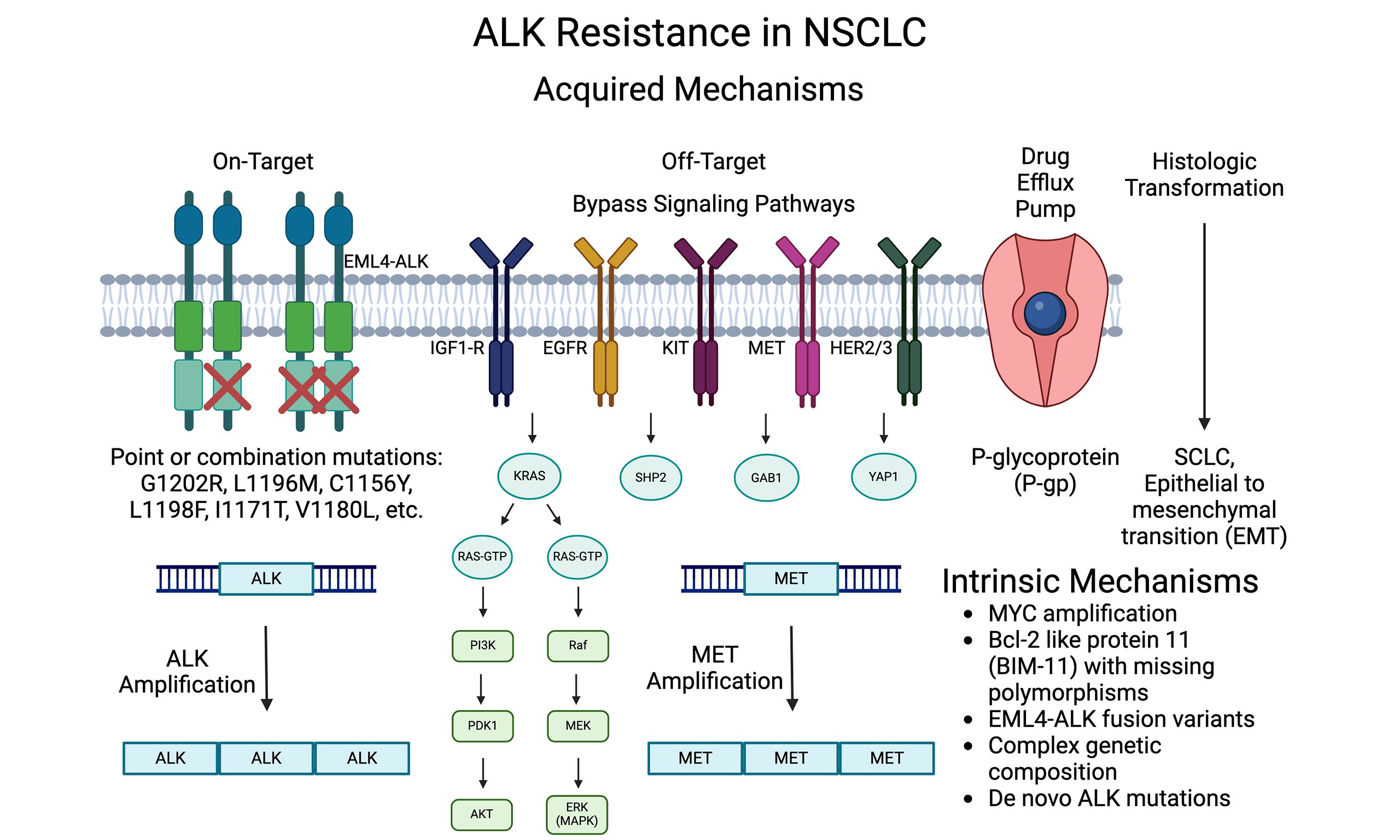
Fig.1 ALK inhibitors in cancer: mechanisms of resistance
What is ALK-Positive Non-Small Cell Lung Cancer?
Non-small cell lung cancer (NSCLC) is a category of lung cancer that includes several types of cancer, including adenocarcinoma and squamous cell carcinoma. ALK-positive NSCLC is a subtype in which the ALK gene undergoes a mutation, causing abnormal growth of cancer cells. In this condition, the ALK protein drives uncontrolled cell division, leading to cancer growth.
ALK mutations can occur in non-smokers and younger adults, which makes it different from many other forms of lung cancer that are more prevalent in smokers. Testing for ALK mutations is a crucial part of diagnosing NSCLC, as it determines whether targeted therapies like Ensartinib can be an effective treatment option.
ALK-positive tumors tend to be more aggressive and harder to treat compared to other types of lung cancer, but the advent of targeted therapies has revolutionized the landscape of treatment options.
What is Ensartinib and How Does it Work?
Ensartinib is a targeted therapy drug, specifically designed to treat patients with ALK-positive NSCLC. Unlike traditional chemotherapy, which indiscriminately attacks both cancerous and healthy cells, targeted therapies focus on specific molecular targets involved in cancer growth. Ensartinib, as an oral ALK inhibitor, works by selectively blocking the ALK protein—the culprit behind cancer cell proliferation.

Fig.2 What is Ensartinib
By inhibiting the ALK pathway, Ensartinib prevents the abnormal signaling that causes cancer cells to divide and grow, effectively halting the progression of the disease. This precise targeting makes it much more effective in treating ALK-positive tumors compared to traditional chemotherapies, which can often have harsher side effects.
One of Ensartinib’s standout features is its ability to target not only the ALK protein but also its mutant variants found in cancer cells. This makes it particularly useful for patients who have developed resistance to other treatments, such as Crizotinib, another ALK inhibitor.
Clinical Trial Results: Efficacy of Ensartinib
The clinical performance of Ensartinib was rigorously tested in the eXALT3 Phase 3 trial, which compared Ensartinib to Crizotinib in patients with advanced or metastatic ALK-positive NSCLC. The results were striking. Patients treated with Ensartinib experienced a significant improvement in progression-free survival (PFS) compared to those on Crizotinib.
Median Progression-Free Survival (PFS): Patients treated with Ensartinib had a PFS of 25.8 months, while those on Crizotinib experienced just 12.7 months. This marked difference suggests that Ensartinib is significantly more effective at slowing disease progression.
CNS Metastasis Response: One of the most impressive findings was Ensartinib’s ability to address brain metastases, which are common in ALK-positive NSCLC. Ensartinib achieved a 63.6% response rate in patients with brain metastases, far surpassing Crizotinib’s 21.2%. This is a major breakthrough, as brain metastases often complicate treatment options for lung cancer patients.
These results are a testament to Ensartinib’s power as a next-generation treatment for a particularly hard-to-treat cancer subtype.
Safety and Side Effects of Ensartinib
As with any medication, Ensartinib comes with potential side effects. However, its tolerability is generally better than traditional chemotherapy options, and most side effects are manageable.
Common Side Effects include:
- Rash (67.8%)
- Musculoskeletal pain (including muscle cramps and joint pain)
- Elevated liver enzymes, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
- Nausea (22.4%)
- Swelling or edema (21.0%)
While these side effects can be bothersome, they are typically manageable through dose adjustments or temporary interruptions in treatment.
More serious adverse effects can occur, although they are rare. These may include:
- Liver toxicity, such as elevated bilirubin or liver enzyme levels
- Pneumonitis, which is inflammation of the lungs
- Hyponatremia (low sodium levels)
Fortunately, these effects can often be mitigated through close monitoring and prompt intervention. For most patients, the benefits of Ensartinib far outweigh the risks, especially in advanced stages of ALK-positive NSCLC where treatment options are limited.
The Future of Ensartinib and Targeted Therapies in Lung Cancer
Ensartinib’s approval marks a significant step forward in the treatment of ALK-positive NSCLC. However, this is just the beginning. Ongoing research is exploring additional indications for Ensartinib, including its potential use in treating other cancers that harbor ALK mutations.
Additionally, Ensartinib’s efficacy in addressing brain metastases opens up exciting possibilities for its use in treating cancers that often spread to the brain. Researchers are optimistic that Ensartinib, along with other targeted therapies, will continue to improve survival rates and quality of life for cancer patients.
As personalized medicine becomes increasingly integrated into cancer treatment, Ensartinib is a key example of how tailored therapies can lead to better outcomes with fewer side effects.
Conclusion
Ensartinib is a groundbreaking medication for treating ALK-positive NSCLC, offering patients a more effective and targeted option compared to traditional treatments. The impressive clinical trial results, particularly its ability to treat brain metastases, make Ensartinib a promising choice for patients struggling with advanced lung cancer.
For anyone diagnosed with ALK-positive NSCLC, Ensartinib represents a significant step forward in treatment. As research continues and more treatment options emerge, the future of targeted therapies in oncology looks brighter than ever.
If you or a loved one has been diagnosed with ALK-positive NSCLC, be sure to consult with your healthcare provider about the most appropriate treatment options, including the potential benefits of Ensartinib.
References
- Yuan X, Wang Y, Yang M, et al. (2023). A retrospective study of ensartinib-treated ALK-positive locally advanced or metastatic NSCLC patients in China. Lung Cancer Manag, 12(4), LMT61. DOI
- Wu Y, Huang L, Li W, Chai Y. (2023). Neoadjuvant target therapy with ensartinib in lung adenocarcinoma with EML4-ALK fusion variant: a case report and literature review. Anticancer Drugs, 34(5), 699-706. DOI
- Zheng J, Wang T, Yang Y, et al. (2024). Updated overall survival and circulating tumor DNA analysis of ensartinib for crizotinib-refractory ALK-positive NSCLC from a phase II study. Cancer Commun (Lond), 44(4), 455-468. DOI
- Rizzo A. (2023). Bayesian analysis supports the role of alectinib and ensartinib for ALK-positive non-small cell lung cancer. Thorac Cancer, 14(13), 1217. DOI




