Exploring the Impact of Hydroxyzine on COVID-19 Mortality: A Groundbreaking Study
Abstract
The COVID-19 pandemic has prompted an urgent search for effective treatments, and recent research highlights the potential of hydroxyzine, a widely used antihistamine, in reducing COVID-19 mortality. A multicenter observational study conducted across 36 hospitals in Greater Paris involving 15,103 hospitalized adults found that hydroxyzine administration within the first 48 hours of hospitalization significantly lowered mortality rates (AOR, 0.51; 95% CI, 0.29–0.88, p = 0.016). Hydroxyzine’s mechanisms include functional inhibition of acid sphingomyelinase (FIASMA) and anti-inflammatory properties, which may mitigate severe inflammatory responses in COVID-19 patients. The study underscores the importance of further investigation through randomized clinical trials to confirm these findings and explore the therapeutic potential of hydroxyzine and its deuterated form, hydroxyzine-d8. The promising results advocate for the repurposing of existing medications to accelerate the availability of effective treatments during public health crises.
Introduction
The COVID-19 pandemic has relentlessly swept across the globe, leaving a trail of devastation in its wake. As scientists and medical professionals race against time to find effective treatments, a recent multicenter observational study has shed light on a promising candidate: hydroxyzine. This widely used antihistamine, known for its antiviral and anti-inflammatory properties, has shown a significant association with reduced mortality in COVID-19 patients.
Conducted across 36 hospitals in Greater Paris, the study involved 15,103 adults hospitalized due to COVID-19. Out of these, 164 patients received hydroxyzine within the first 48 hours of their hospital stay. The findings revealed a remarkable outcome: patients who were administered hydroxyzine had a notably lower mortality rate compared to those who did not receive the drug. Specifically, the analysis demonstrated a significant association between hydroxyzine use and reduced mortality, with an adjusted odds ratio (AOR) of 0.51 (95% CI, 0.29–0.88, p = 0.016).
Hydroxyzine’s potential mechanisms of action in combating COVID-19 are multifaceted. It functions as a functional inhibitor of acid sphingomyelinase (FIASMA), which may contribute to its antiviral effects. Additionally, its anti-inflammatory properties, achieved through both receptor-dependent and receptor-independent mechanisms, could play a crucial role in mitigating the severe inflammatory response seen in COVID-19 patients.
Despite the study’s observational nature, which inherently carries the risk of biases, the results are compelling and warrant further investigation through randomized clinical trials. The findings suggest that hydroxyzine could be a valuable addition to the arsenal of treatments against COVID-19, potentially saving numerous lives.
As we continue to navigate through the challenges posed by the pandemic, studies like this offer a glimmer of hope and underscore the importance of exploring existing medications for new therapeutic purposes.
Background Information
The COVID-19 pandemic has necessitated the rapid development and identification of effective treatments to mitigate its impact. COVID-19, caused by the SARS-CoV-2 virus, has resulted in significant morbidity and mortality worldwide, prompting an urgent need for therapeutic interventions. While vaccines have proven to be a critical tool in combating the virus, the search for effective treatments remains a priority, particularly for severe cases.
Hydroxyzine, a first-generation H1 antihistamine, is commonly used to treat conditions such as urticaria, allergic rhinitis, hay fever, conjunctivitis, and pruritus. Beyond its primary antihistaminic activity, hydroxyzine is also recognized for its tranquilizer and sedative properties. The drug exerts its effects by competitively binding to H1 receptors, thereby inhibiting the action of histamine—a key mediator in allergic inflammatory responses.
Recent studies have highlighted additional anti-inflammatory properties of hydroxyzine, including both receptor-dependent and receptor-independent mechanisms. The receptor-dependent mechanisms involve the inhibition of NF-kB-dependent cytokines such as IL-1, IL-2, IL-6, IL-8, IL-12, and TNF-α, as well as adhesion proteins like ICAM-1 and VCAM-1. These mechanisms are crucial in controlling the excessive inflammatory responses seen in severe COVID-19 cases.
Moreover, hydroxyzine belongs to a group of drugs known as functional inhibitors of acid sphingomyelinase (FIASMA). This group includes various medications such as antidepressants, calcium channel blockers, and mucolytics. These drugs inhibit the acid sphingomyelinase enzyme, which is implicated in the SARS-CoV-2 infection process by facilitating viral entry into host cells. This inhibition potentially reduces the severity of the infection and the associated inflammatory response.
Given these properties, hydroxyzine emerged as a candidate for repurposing in the treatment of COVID-19. The multicenter observational study conducted across 36 hospitals in Greater Paris aimed to explore this potential, revealing a significant association between hydroxyzine use and reduced mortality in hospitalized COVID-19 patients.
Analysis and Implications
The findings from the multicenter observational study on hydroxyzine use in COVID-19 patients provide significant insights into potential therapeutic avenues. The study revealed that hydroxyzine, when administered within the first 48 hours of hospitalization, was associated with a substantial reduction in mortality. This association held even after adjusting for various confounding factors, indicating a strong potential for hydroxyzine to play a beneficial role in the treatment of COVID-19.
One of the key mechanisms through which hydroxyzine may exert its effects is its action as a functional inhibitor of acid sphingomyelinase (FIASMA). By inhibiting this enzyme, hydroxyzine could potentially disrupt the SARS-CoV-2 infection process, thereby reducing viral entry and subsequent cellular damage. This mechanism is supported by previous research demonstrating the antiviral effects of FIASMA medications against other viruses, including hepatitis C and MERS.
Additionally, hydroxyzine’s anti-inflammatory properties are noteworthy. The drug’s ability to inhibit NF-kB-dependent cytokines and adhesion proteins suggests it could mitigate the severe inflammatory response often observed in COVID-19 patients. This anti-inflammatory action could be crucial in preventing the progression of mild to severe disease, particularly by controlling the cytokine storm associated with severe COVID-19 cases.
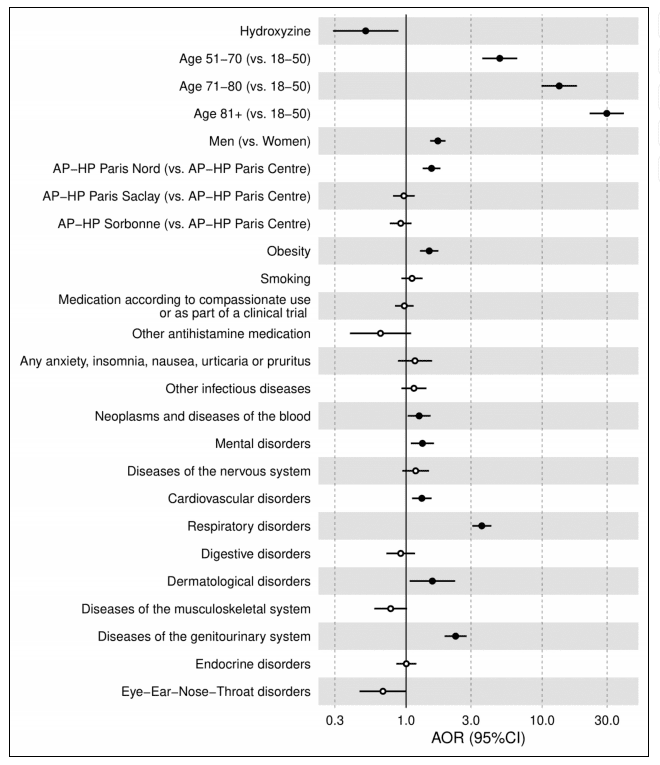
However, the observational nature of the study inherently includes potential biases and confounding factors, despite the multivariable adjustments made. These limitations underscore the need for randomized controlled trials to definitively ascertain the efficacy and safety of hydroxyzine in COVID-19 treatment. Such trials would help to eliminate biases and provide more robust evidence of hydroxyzine’s therapeutic potential.
The study’s implications extend beyond immediate clinical applications. It highlights the importance of drug repurposing in the rapid response to emerging infectious diseases. The findings encourage further exploration of existing medications with known safety profiles, potentially accelerating the availability of effective treatments during public health crises.
Predicted Effects of Hydroxyzine-d8 on COVID-19 Mortality
The potential application of hydroxyzine-d8, a deuterated form of hydroxyzine, in the treatment of COVID-19 warrants attention based on the promising findings from the recent multicenter observational study. Hydroxyzine-d8 is chemically modified to contain deuterium atoms, which replace some of the hydrogen atoms in the molecule. This modification can lead to improved metabolic stability and a prolonged half-life, potentially enhancing the drug’s therapeutic efficacy.
Given the study’s findings that hydroxyzine is associated with reduced mortality in hospitalized COVID-19 patients, it is reasonable to predict that hydroxyzine-d8 could offer similar, if not superior, benefits. The enhanced metabolic stability of hydroxyzine-d8 might allow for more consistent therapeutic levels in the bloodstream, potentially leading to a more sustained antiviral and anti-inflammatory effect. This could be particularly beneficial in managing the severe inflammatory response and cytokine storm associated with COVID-19.
Furthermore, the functional inhibition of acid sphingomyelinase (FIASMA) by hydroxyzine plays a crucial role in its antiviral effects. Hydroxyzine-d8 is expected to maintain this mechanism of action, possibly with enhanced efficacy due to its improved pharmacokinetic profile. The inhibition of acid sphingomyelinase could further prevent SARS-CoV-2 from entering host cells, thereby reducing viral load and mitigating the severity of the infection.
However, it is essential to conduct specific studies on hydroxyzine-d8 to validate these predictions. While the existing study provides a strong foundation, clinical trials specifically examining hydroxyzine-d8’s effects on COVID-19 patients would be necessary to confirm its safety and efficacy. These trials should assess not only mortality rates but also other critical outcomes such as hospitalization duration, ICU admissions, and overall recovery rates.
In conclusion, hydroxyzine-d8 holds potential as a therapeutic agent for COVID-19, building on the encouraging results observed with hydroxyzine. Its predicted effects, including enhanced stability and sustained therapeutic action, could make it a valuable addition to the COVID-19 treatment arsenal, pending further research.
Conclusion
The recent multicenter observational study investigating hydroxyzine’s effect on COVID-19 mortality offers promising insights into the potential repurposing of existing medications to combat this global pandemic. The study demonstrated a significant association between hydroxyzine use and reduced mortality among hospitalized COVID-19 patients, suggesting that this widely used antihistamine could play a beneficial role in the treatment of severe COVID-19 cases. These findings are particularly compelling given hydroxyzine’s well-documented safety profile and its additional anti-inflammatory and antiviral properties.
Hydroxyzine’s mechanisms of action, including functional inhibition of acid sphingomyelinase (FIASMA) and suppression of pro-inflammatory cytokines, provide a strong theoretical basis for its effectiveness against COVID-19. The potential for hydroxyzine-d8, a deuterated variant of the drug, to offer enhanced stability and sustained therapeutic levels further underscores the need for continued research in this area. By extending the drug’s half-life, hydroxyzine-d8 could provide even more consistent antiviral and anti-inflammatory effects, potentially improving patient outcomes further.
Despite the observational nature of the study, which introduces inherent limitations such as potential biases and confounding factors, the results are robust enough to warrant further investigation. Randomized controlled trials (RCTs) are essential to confirm these findings and establish clear clinical guidelines for hydroxyzine use in COVID-19 treatment. Such trials will help eliminate biases and provide definitive evidence of hydroxyzine’s efficacy and safety in this new therapeutic context.
In conclusion, the study on hydroxyzine offers a glimmer of hope in the ongoing fight against COVID-19. It highlights the importance of exploring and repurposing existing medications, which can be rapidly deployed to treat new and emerging infectious diseases. As the global scientific community continues to battle this pandemic, the potential role of hydroxyzine and similar drugs should be a focal point for future research efforts.
References
- Hoertel, N., Sánchez-Rico, M., Gulbins, E., Kornhuber, J., Carpinteiro, A., Lenze, E. J., … & AP-HP/Université de Paris/INSERM COVID-19 Research Collaboration. (2021). Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID-19: An observational multicenter study. Clinical Pharmacology & Therapeutics, 110(6), 1498-1511.
- Kornhuber, J., Hoertel, N., & Gulbins, E. (2021). The acid sphingomyelinase/ceramide system in COVID-19. Molecular Psychiatry, 26(7), 2794-2795.
- Marín-Corral, J., Rodríguez-Morató, J., Gomez-Gomez, A., Pascual-Guardia, S., Muñoz-Bermúdez, R., Salazar-Degracia, A., … & de la Muela, P. (2021). Metabolic signatures associated with severity in hospitalized COVID-19 patients. International Journal of Molecular Sciences, 22(9), 4794.
- Sánchez-Rico, M., Limosin, F., Vernet, R., Beeker, N., Neuraz, A., Blanco, C., … & Hoertel, N. (2021). Hydroxyzine use and mortality in patients hospitalized for COVID-19: A multicenter observational study. Journal of Clinical Medicine, 10(24), 5891.
- Kornhuber, J., Muehlbacher, M., Trapp, S., Pechmann, S., Friedl, A., Reichel, M., … & Gulbins, E. (2011). Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE, 6(8), e23852.
- Mandhane, S. N., Shah, J. H., Bahekar, P. C., Mehetre, S. V., Pawar, C. A., Bagad, A. S., … & Rajamannar, T. (2010). Characterization of anti-inflammatory properties and evidence for no sedation liability for the novel antihistamine SUN-1334H. International Archives of Allergy and Immunology, 151(1), 56-69.
- Carpinteiro, A., Edwards, M. J., Hoffmann, M., Kochs, G., Gripp, B., Weigang, S., … & Gulbins, E. (2020). Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. Cell Reports Medicine, 1(8), 100142.