Exploring the Role of Toll-like Receptor (TLR) Agonists in Cancer Therapy
Abstract
Cancer treatment has advanced significantly with the advent of immunotherapy, which utilizes the body’s immune system to target and eliminate cancer cells. Among the innovative strategies in immunotherapy, Toll-like receptor (TLR) agonists have emerged as promising therapeutic agents. TLRs, essential for innate immune responses, recognize pathogen-associated and damage-associated molecular patterns, thereby activating immune cells and initiating robust immune reactions. The application of TLR agonists in cancer therapy aims to convert immunologically “cold” tumors into “hot” tumors, enhancing their susceptibility to immune checkpoint inhibitors (ICIs). This approach offers new hope for patients with resistant or refractory cancers. Current clinical trials are investigating the efficacy and safety of various TLR agonists, either as standalone treatments or in combination with other therapies. The ongoing research seeks to optimize these therapies to improve patient outcomes and minimize adverse effects, potentially revolutionizing cancer treatment.
Introduction to Toll-like receptors (TLRs)
Cancer remains one of the most challenging diseases to treat, with traditional therapies like chemotherapy and radiation often falling short due to their inability to distinguish between healthy and cancerous cells, leading to significant side effects. In recent years, the advent of immunotherapy has revolutionized cancer treatment by harnessing the body’s immune system to specifically target and destroy cancer cells. Among the various strategies in immunotherapy, the use of Toll-like receptor (TLR) agonists has gained considerable attention.
Toll-like receptors (TLRs) are a class of proteins that play a crucial role in the immune system. They function as sentinels, recognizing pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) to initiate and regulate immune responses. TLRs are expressed on various immune cells, including dendritic cells, macrophages, and T cells, and their activation leads to the production of cytokines and other mediators that orchestrate the body’s defense mechanisms.
The therapeutic potential of TLR agonists in cancer is based on their ability to stimulate the immune system, enhancing the recognition and destruction of tumor cells. By activating innate immunity and bridging it with adaptive immune responses, TLR agonists can convert immunologically “cold” tumors, which typically evade immune detection, into “hot” tumors that are more likely to respond to immunotherapy, such as immune checkpoint inhibitors (ICIs). This conversion is pivotal as it can make previously unresponsive tumors susceptible to treatment, offering new hope for patients with resistant or refractory cancers.
In this context, the exploration of TLR agonists represents a promising frontier in cancer therapy. Clinical trials are currently investigating various TLR agonists, either as monotherapies or in combination with other treatments, to evaluate their safety and efficacy. The ongoing research aims to optimize these therapies, reduce side effects, and improve patient outcomes, heralding a new era in cancer treatment.
Understanding Toll-like Receptors (TLRs)
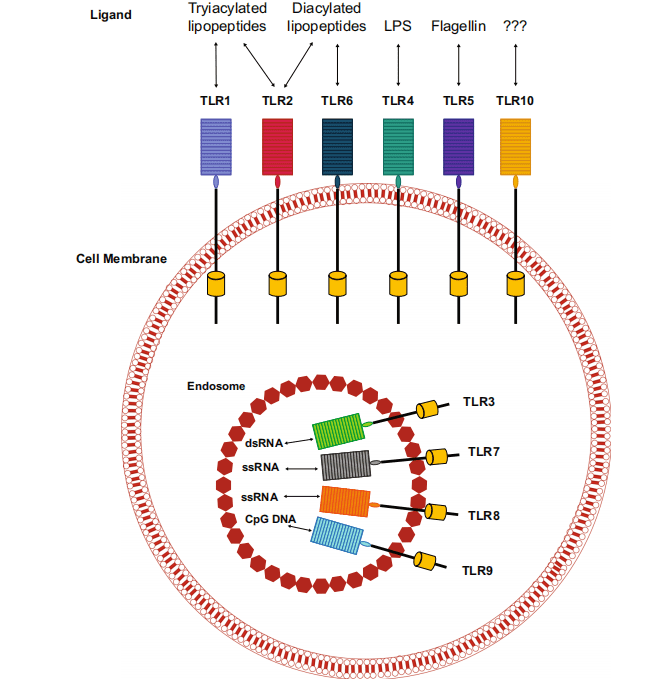
Toll-like receptors (TLRs) are a class of proteins that play a critical role in the immune system. They function as the first line of defense against pathogens by recognizing pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). These patterns are typically found on microbial surfaces and are released by damaged or dying cells, respectively. TLRs are crucial for initiating the immune response and bridging innate and adaptive immunity.
Structurally, TLRs are transmembrane proteins found on various immune cells, including macrophages, dendritic cells, and lymphocytes, as well as non-immune cells like epithelial cells and fibroblasts. In humans, there are ten known TLRs, each recognizing specific PAMPs and DAMPs. For example, TLR4 recognizes bacterial lipopolysaccharide (LPS), while TLR3 detects double-stranded RNA, a molecular signature of viral infections.
Upon recognizing these molecular patterns, TLRs trigger signaling cascades that result in the activation and maturation of antigen-presenting cells, such as macrophages and dendritic cells. This activation leads to the secretion of pro-inflammatory cytokines and the presentation of antigens to naïve T lymphocytes. The interaction between TLRs and these immune cells promotes the differentiation of T cells into effector cells that are capable of targeting and eliminating pathogens or tumor cells.
The role of TLRs in cancer is multifaceted. They can contribute to both tumor suppression and progression, depending on the context. TLR activation can enhance anti-tumor immunity by stimulating the production of inflammatory cytokines and promoting tumor cell death. However, chronic TLR activation can also lead to a tumor-promoting environment by facilitating cancer cell proliferation, survival, and metastasis. Understanding the dual nature of TLRs in cancer biology is essential for developing targeted therapies that harness their beneficial effects while minimizing potential adverse outcomes.
TLRs and Their Impact on Cancer
Toll-like receptors (TLRs) have a complex and dualistic role in cancer, influencing both tumor progression and suppression. These receptors, crucial for immune system activation, are often overexpressed in various cancer types, including pancreatic, esophageal, lung, and ovarian cancers. TLRs recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), triggering immune responses that can impact tumor dynamics.
In cancer progression, certain TLRs may create a tumor-promoting environment. For instance, TLR4 activation has been associated with promoting cancer cell proliferation, survival, and metastasis. This pro-tumor effect is mediated through the secretion of anti-apoptotic factors and the induction of a chronic inflammatory state, which supports tumor growth and protects tumor cells from immune attack. Similarly, TLR5 and TLR9 have been implicated in enhancing tumor cell survival and proliferation through various signaling pathways.
Conversely, TLRs also have significant roles in tumor suppression. TLR activation can enhance anti-tumor immunity by stimulating the production of pro-inflammatory cytokines and chemokines, which recruit and activate cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells to the tumor site. For example, TLR7 and TLR8 activation has shown potential in reducing tumor burden by promoting immune-mediated tumor cell destruction. The stimulation of these receptors can convert “cold” tumors, which are less responsive to immunotherapy, into “hot” tumors with heightened immunogenicity, making them more susceptible to immune checkpoint inhibitors (ICIs).
The intricate balance between the tumor-promoting and tumor-suppressing roles of TLRs is influenced by various factors, including the type of TLR, the specific cancer context, and the overall tumor microenvironment. Understanding these dynamics is crucial for developing effective cancer therapies that leverage TLRs’ beneficial effects while mitigating their potential to support tumor growth. As research advances, targeting TLR pathways offers a promising strategy to enhance cancer treatment efficacy and improve patient outcomes.
TLR Agonists in Cancer Therapy
Toll-like receptor (TLR) agonists represent a promising avenue in cancer therapy, leveraging the body’s innate immune system to combat tumor cells. These agonists work by mimicking natural ligands that activate TLRs, thereby initiating robust immune responses against cancer. By targeting specific TLRs, these agents can stimulate the production of pro-inflammatory cytokines and enhance the activity of cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, which are essential for effective anti-tumor immunity.
One of the most well-known TLR agonists is Imiquimod, a TLR7 agonist approved by the FDA for treating superficial basal cell carcinoma and actinic keratosis. Imiquimod activates immune cells by binding to TLR7, leading to the release of cytokines such as interferon-alpha (IFN-α), which plays a critical role in anti-tumor responses. Clinical studies have shown that Imiquimod not only reduces tumor size but also enhances the overall immune environment within the tumor, making it more susceptible to further immune attack.
Beyond Imiquimod, other TLR agonists are in various stages of clinical development. For instance, TLR7/8 agonists like Resiquimod and NKTR-262 are being investigated for their ability to stimulate strong anti-tumor immunity. These agonists have demonstrated potential in preclinical models, where they have reduced tumor burden and improved survival rates when used alone or in combination with other immunotherapies, such as immune checkpoint inhibitors (ICIs).
TLR9 agonists, such as Lefitolimod and CMP-001, are also being explored. TLR9 recognizes unmethylated CpG motifs commonly found in bacterial DNA, triggering immune responses that can enhance the body’s ability to target and destroy cancer cells. Early clinical trials have indicated that TLR9 agonists can induce significant anti-tumor activity, particularly when used in combination with ICIs, providing a synergistic effect that enhances overall treatment efficacy.
The clinical application of TLR agonists in cancer therapy offers a novel and potent strategy to enhance immune responses against tumors. By converting immunologically “cold” tumors into “hot” tumors, TLR agonists can make cancer cells more recognizable and attackable by the immune system, potentially improving outcomes for patients who do not respond to conventional therapies.
Clinical Trials and Results
Clinical trials investigating Toll-like receptor (TLR) agonists in cancer therapy have shown promising results, highlighting their potential to enhance anti-tumor immunity and improve patient outcomes. These trials have primarily focused on TLR7, TLR7/8, and TLR9 agonists, evaluating their efficacy as monotherapies and in combination with other treatments, such as immune checkpoint inhibitors (ICIs).
TLR7 agonists, such as Imiquimod, have demonstrated significant clinical benefits in treating skin cancers like superficial basal cell carcinoma. Clinical studies have shown that Imiquimod induces local immune responses that result in tumor regression. Another TLR7 agonist, TQ-A3334, is currently under investigation for non-small-cell lung cancer (NSCLC). Early phase trials have indicated that TQ-A3334 is well-tolerated and capable of inducing pro-inflammatory cytokines, which are crucial for anti-tumor activity.
TLR7/8 agonists, like BDB001 and NKTR-262, are being tested for their ability to enhance immune responses against solid tumors. In clinical trials, BDB001 has shown potential in combination with pembrolizumab, an anti-PD-1 antibody, by inducing cytokine production and promoting the infiltration of immune cells into the tumor microenvironment. This combination has resulted in partial responses and stable disease in a significant number of patients with advanced solid tumors. Similarly, NKTR-262, when used with another immunotherapeutic agent, bempegaldesleukin, has shown enhanced anti-tumor effects in preclinical studies, leading to ongoing clinical trials to evaluate its efficacy in humans.
TLR9 agonists, such as Lefitolimod and CMP-001, have also shown encouraging results. Lefitolimod, a TLR9 agonist, has been tested in combination with ipilimumab, an anti-CTLA-4 antibody, in patients with advanced solid tumors. Preliminary results indicate that this combination can activate immune responses and induce tumor regression. CMP-001, another TLR9 agonist, has been studied in combination with pembrolizumab for melanoma treatment. Clinical trials have demonstrated that CMP-001 can induce responses in patients who have previously progressed on anti-PD-1 therapies, showcasing its potential to overcome resistance to existing treatments.
Overall, these clinical trials underscore the potential of TLR agonists to significantly impact cancer therapy by enhancing immune responses and improving the efficacy of existing treatments. The ongoing research and development of these agents continue to provide hope for more effective cancer treatments in the future.
Combining TLR Agonists with Other Therapies
Combining Toll-like receptor (TLR) agonists with other cancer therapies, particularly immune checkpoint inhibitors (ICIs), has emerged as a promising strategy to enhance anti-tumor immune responses and improve treatment outcomes. TLR agonists can convert “cold” tumors, which are immunologically inert and less responsive to immunotherapy, into “hot” tumors that exhibit robust immune activity, making them more susceptible to ICIs.
Immune checkpoint inhibitors, such as pembrolizumab (anti-PD-1) and ipilimumab (anti-CTLA-4), have revolutionized cancer treatment by blocking inhibitory pathways that prevent T cells from attacking tumor cells. However, not all patients respond to ICIs alone. Combining ICIs with TLR agonists can potentially overcome this limitation by enhancing the overall immune response. TLR agonists activate innate immune pathways, leading to the production of pro-inflammatory cytokines and the activation of antigen-presenting cells. This results in the increased infiltration and activation of cytotoxic T lymphocytes (CTLs) within the tumor microenvironment.
Clinical trials have demonstrated the potential benefits of such combinations. For instance, the TLR7/8 agonist NKTR-262, when combined with bempegaldesleukin, a CD122-preferential IL-2 pathway agonist, showed enhanced anti-tumor activity in preclinical models. This combination is currently being evaluated in clinical trials, with early results indicating improved immune activation and tumor regression.
Similarly, the TLR9 agonist CMP-001 has been combined with pembrolizumab in patients with melanoma. This combination has shown promising results, with some patients experiencing tumor regression despite prior resistance to anti-PD-1 therapy. The synergistic effect observed with CMP-001 and pembrolizumab highlights the potential of TLR agonists to improve the efficacy of ICIs.
Another example is the combination of the TLR7 agonist Imiquimod with ICIs. Imiquimod has been used topically in conjunction with systemic ICIs to treat skin cancers. The localized activation of the immune response by Imiquimod complements the systemic effects of ICIs, resulting in more effective tumor control.
The combination of TLR agonists with other therapies represents a promising frontier in cancer treatment. By leveraging the innate and adaptive immune responses, these combinations can potentially enhance the efficacy of existing therapies, offering new hope for patients with resistant or refractory cancers.
Future Directions and Conclusion
The future of cancer therapy with Toll-like receptor (TLR) agonists looks promising, with ongoing research aiming to enhance their efficacy and reduce side effects. One of the key areas of focus is understanding the precise mechanisms by which TLR agonists modulate the immune system and identifying biomarkers that can predict patient response. This knowledge will enable the development of more targeted therapies and improve patient outcomes.
Combining TLR agonists with other immunotherapies, such as immune checkpoint inhibitors (ICIs), continues to be a significant area of investigation. This combination strategy aims to harness the synergistic effects of both treatments, thereby overcoming resistance seen with monotherapies. Clinical trials are exploring various combinations, dosing regimens, and administration routes to optimize the anti-tumor response while minimizing adverse effects.
Personalized medicine is another frontier in the application of TLR agonists. By tailoring treatments based on individual patient profiles, including genetic, immunological, and tumor-specific characteristics, clinicians can improve the effectiveness of TLR-based therapies. Advances in genomic and proteomic technologies will play a crucial role in this personalized approach, enabling the identification of patients who are most likely to benefit from TLR agonists.
Furthermore, ongoing research is exploring new TLR agonists and novel delivery systems. These efforts aim to increase the specificity and potency of TLR agonists, reduce systemic toxicity, and enhance their ability to target tumor cells directly. Nanotechnology and other innovative delivery platforms are being investigated to achieve these goals.
In conclusion, TLR agonists hold significant potential in cancer therapy, offering new hope for enhancing immune responses and improving patient outcomes. While challenges remain, particularly regarding safety and patient selection, the future of TLR-based therapies is bright. Continued research and clinical trials will pave the way for more effective and personalized cancer treatments, making a meaningful impact on the lives of patients.
References
- Akira, S., & Takeda, K. (2004). Toll-like receptor signalling. Nature Reviews Immunology, 4(7), 499-511.
- Kawai, T., & Akira, S. (2010). The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunology, 11(5), 373-384.
- Medzhitov, R. (2001). Toll-like receptors and innate immunity. Nature Reviews Immunology, 1(2), 135-145.
- Rakoff-Nahoum, S., & Medzhitov, R. (2009). Toll-like receptors and cancer. Nature Reviews Cancer, 9(1), 57-63.
- Takeda, K., & Akira, S. (2005). Toll-like receptors in innate immunity. International Immunology, 17(1), 1-14.
- Adams, S. (2009). Toll-like receptor agonists in cancer therapy. Immunotherapy, 1(6), 949-964.
- Krieg, A. M., Yi, A. K., Matson, S., Waldschmidt, T. J., Bishop, G. A., Teasdale, R., … & Klinman, D. M. (1998). CpG motifs in bacterial DNA trigger direct B-cell activation. The Journal of Immunology, 161(3), 186-193.
- Lu, H. (2014). TLR agonists for cancer immunotherapy: Tipping the balance between the immune stimulatory and inhibitory effects. Frontiers in Immunology, 5, 83.
- Krieg, A. M. (2008). Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene, 27(2), 161-167.
- Ribas, A., & Wolchok, J. D. (2018). Cancer immunotherapy using checkpoint blockade. Science, 359(6382), 1350-1355.
- Rolfo, C., Giovannetti, E., Martinez, P., McCue, S., & Naing, A. (2023). Applications and clinical trial landscape using Toll-like receptor agonists to reduce the toll of cancer. Journal of Experimental & Clinical Cancer Research, 42, Article 35.