Extracellular Protein Targeted Degradation (eTPD): Six Major Drug Discovery Patterns
Abstract
Targeted Protein Degradation (TPD) has emerged as a major novel drug modality in the past decade, used to eliminate proteins with dual-specific small molecules inside cells. These small molecules recruit the protein of interest (POI) to the E3 ligase, facilitating degradation in the proteasome. Unlike traditional occupancy-based drugs, intracellular TPD (iTPD) eliminates the target and catalyzes its action, making it more efficient and sustained, with lower dosage requirements. Recently, this approach has been extended to extracellular proteomes, including secreted and membrane proteins. Extracellular Protein Targeted Degradation (eTPD) utilizes dual-specific antibodies, conjugates, or small molecules to transport extracellular POI to lysosomes for degradation. Here, we focus on the latest advancements in eTPD, including degradation systems, targets, molecular design, and parameters driving their progress.
Intracellular Protein Targeted Degradation (iTPD): PROTAC and Molecular Glue
In recent years, iTPD has emerged as a significant new mode for small molecule drugs. Currently, two classes of molecules have been developed. The first is PROTACs, large dual-specificity small molecules connected by a linker that induce the ubiquitination of the Protein of Interest (POI), leading to its degradation in the cellular proteasome. The second class is referred to as molecular glues, which enhance the interaction between E3 ligase and POI, resulting in ubiquitination and subsequent proteasomal degradation. In comparison to traditional “occupancy-driven” inhibitors, iTPD has notable advantages. Firstly, PROTACs or molecular glues can theoretically bind anywhere on the POI, while inhibitors typically require binding to active or allosteric sites. Secondly, the degradation of the target removes the entire protein, including its scaffold function, allowing for a longer duration of therapeutic activity, closer to the results of gene knockout experiments. Thirdly, these molecules act catalytically, unlike classical pharmacological inhibitors, which are stoichiometric, enabling equivalent therapeutic effects at lower doses. Currently, there are over twenty clinical trials involving PROTACs and molecular glues, with some progressing to Phase II and Phase III studies.
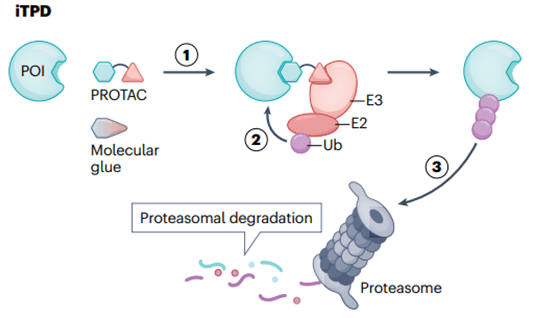
Fig 1. General Mechanism of Intracellular Targeted Protein Degradation Pathway
Extracellular Protein Targeted Degradation (eTPD): Emerging Drug Modality
eTPD refers to the study of the extracellular proteome in biology and chemistry. Apart from the location of the target POI, there are at least three significant differences between iTPD and eTPD (Fig 2).
Firstly, cells recycle proteins through two main pathways: the proteasomal and lysosomal pathways. iTPD predominantly relies on the proteasomal pathway, which is typically utilized for intracellular proteins. On the other hand, eTPD involves dual-specificity biological agents or small molecules that recruit membrane-bound or secreted POIs to membrane-associated cycling receptors, transporting the POI to lysosomes, the typical pathway for extracellular protein degradation.
Secondly, these two protein hydrolysis degradation mechanisms exhibit different kinetics and employ distinct proteases. iTPD is faster—typically occurring within minutes to hours, as all components are within the cell. In contrast, eTPD is generally slower, taking 6-48 hours, as it involves vesicular transport from the membrane through early and late endosomes, ultimately fusing with lysosomes leading to protein degradation.
Thirdly, nearly all iTPD systems use only a few E3 ligases, primarily CRBN or VHL, presenting challenges in finding E3 ligase binding partners. These ligases are widely expressed in tissues, limiting tissue-selective targeting. In contrast, eTPD can employ multiple degradation systems, allowing for more specific tissue selectivity.
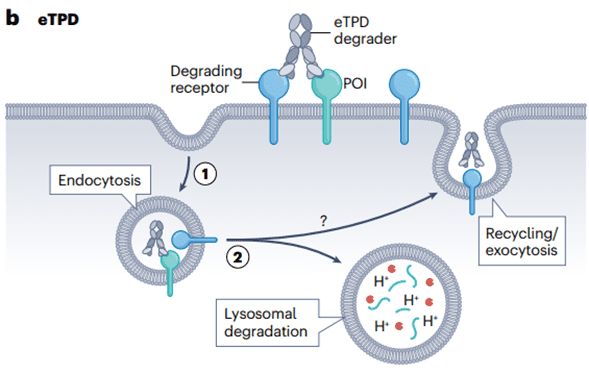
Fig 2. General Mechanism of Extracellular Targeted Protein Degradation Pathway
The six different strategies of eTPD
Clearance Antibodies eTPD for Degradation of Soluble POI
The Fc region of antibodies plays a crucial role in antibody circulation and immune cell activation. The neonatal Fc receptor (FcRn) is responsible for recycling antibodies internalized into endosomes, returning them to the extracellular space before reaching lysosomal degradation. Enhanced binding between antibodies and FcRn can extend their half-life in human serum to 21 days.
“Clearance antibodies” are designed to deliver POI to the acidic endosome in a pH-switchable manner through FcRn, releasing the POI for lysosomal degradation (Fig 3a). This is inspired by the natural cycling mechanism of low-density lipoprotein (LDL) uptake, where LDL binds to LDL receptors and is released into endosomes for lysosomal degradation at low pH. Tocilizumab (Tcz), a humanized antibody approved for treating rheumatoid arthritis, exhibits pH-dependent binding to the IL-6 receptor (IL-6R). By redesigning Tcz into a clearance antibody, its affinity for FcRn is enhanced using known mutations. Subsequently, through mutations in the complementarity-determining region (CDR) at pH 6.0, the affinity for IL-6 is reduced approximately 20-fold, while maintaining affinity at pH 7.4. The modified antibody can transport IL-6R to lysosomes, while maintaining its connection with FcRn, circulating back to the membrane to collect more IL-6. The pH-switchable Tocilizumab was approved in 2020 for treating neuromyelitis optica spectrum disorders, representing the first approved antibody in eTPD.
Glycan-based Circulatory Receptor eTPD
Using polysaccharide-targeting recycling receptors, such as the cation-independent mannose-6-phosphate receptor (CI-M6PR) or asialoglycoprotein receptor (ASGPR), can facilitate lysosomal degradation of both membrane-bound and soluble Proteins of Interest (POI) (Figure 3b). One approach is known as Lysosome-Targeting Chimeras (LYTAC), involving the bioconjugation of multiple polysaccharide ligands of CI-M6PR with antibodies targeting the POI. The LYTAC–POI complex, upon binding to CI-M6PR, undergoes internalization, leading to the degradation of POI in the lysosome. Another approach involves dual-functional small molecules known as Monomers of Degraders (MoDE) or ASGPR-Targeted Chimeras (ATACs), which have been used to develop ASGPR-based methods.
CI-M6PR is a 300 kDa dimeric type I receptor, known as a pH-switchable receptor. It binds to ligands at neutral pH and releases them in acidic endosomes before fusion with lysosomes. CI-M6PR has been utilized to shuttle exogenous lysosomal enzymes carrying M6P to lysosomes, aiding in the treatment of lysosomal diseases. ASGPR is predominantly expressed on hepatocytes and rapidly clears non-sialylated glycoproteins.
eTPD Targeting Membrane Proteins Based on Transmembrane E3 Ligase
In addition to the approximately 600–700 intracellular members, the E3 ligase family also includes a subfamily with a transmembrane structural domain, consisting of about 30 members. Utilizing dual-specific antibodies targeting this subfamily’s members and POI can lead to the degradation of the target protein, known as Antibody-based Targeted Protein Degradation Chimeras (AbTACs), PROtein TABs (PROTABs), or Receptor Elimination Using Ubiquitin Ligase Recruitment (REULR) (Figure 3c). In a study, an AbTAC for PD-L1 was engineered, with the POI arm selecting the Fab domain of atezolizumab that binds to PD-L1, and the degradation arm using the Fab domain of RNF43 extracellular structure. The Atz-AbTAC followed the classical KIH model, inducing the degradation of PD-L1 with a DC50 of 3.4nM, approximately 63% maximum degradation (Dmax) in 24 hours. Whole-cell proteomics showed no significant overall changes in the cellular proteome, and there was no noticeable cytotoxicity.
In summary, existing research indicates that dual-specific molecules targeting transmembrane E3 ubiquitin ligase family members (AbTACs, PROTABs, and REULRs) can collectively select for the degradation of certain crucial membrane proteins, thereby achieving therapeutic goals.
eTPD Targeting Membrane Proteins Based on Cytokines
As is well known, many cellular factors can be absorbed and degraded by their receptors through trafficking to lysosomes. This provides another opportunity to utilize the endogenous mechanism of eTPD, a method known as Cytokine Receptor Targeting Chimeras (KineTAC) (Figure 3d). KineTACs have one arm binding to natural cytokines or growth factors, while the other arm specifically binds to POI. This enables it to effectively and selectively degrade both membrane-bound and soluble proteins.
Similar to AbTAC, KineTAC can degrade a wide range of membrane proteins on various cell types. Additionally, similar to LYTAC, they can also degrade soluble proteins as their circulation does not depend on ubiquitination. Furthermore, the transcription levels of the POI can be significantly higher than that of the degrading substrate, and effective degradation can still occur.
Integrin-based eTP
Inspired by the targeted delivery of anti-cancer drugs utilizing integrin αVβ3, eTPD introduces an integrin-based degradation system (Figure 3e). It involves the use of dual-specific antibodies, with one arm specifically binding to integrin and the other arm binding to POI. This guides the POI to lysosomes for degradation, while utilizing the integrin’s recycling capability to enhance target localization and efficiency.
As a proof of concept, a biotin-conjugated molecule targeting NeutrAvidin through integrin αVβ3 binding was utilized. This molecule contained a cyclic RGD motif (cRGD) covalently linked to biotin, successfully internalizing NeutrAvidin in A549 cells within 20 hours. Overall, this integrin-based degradation system demonstrates exciting potential for the selective degradation of membrane or soluble proteins in tumors.
eTPD for Internal Degradation of Membrane Proteins
Research on Receptor Tyrosine Kinases (RTKs) targeted by PROTACs has been conducted, where the PROTAC contains a classic E3 ligase binder, CRBN or VHL, connected to various RTK inhibitors (Figure 3f). This PROTAC binds to the intracellular kinase domain of the RTK and simultaneously binds to the E3 ligase. As a result, ubiquitination of the receptor tyrosine kinase (RTK) is induced, leading to the degradation of RTK in the proteasome. In summary, PROTACs binding to VHL or CRBN can internally degrade RTK. Although this approach is still in its early stages, the use of PROTACs targeting the intracellular domain of membrane proteins has shown potential applications.
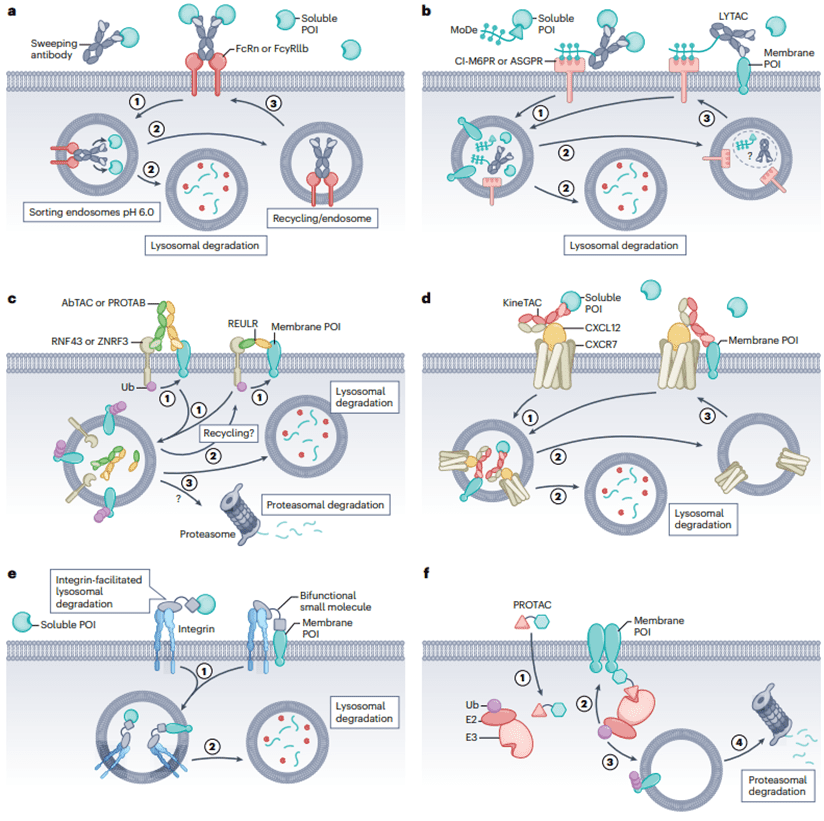
Fig 3. Six Different Methods for Degrading Extracellular Proteins
Summary
The eTPD field is in its early stages, rapidly following the development of the iTPD field. Currently, various designs and methods are under investigation. However, there are still many crucial issues to be addressed in the eTPD field.
In what aspects does eTPD demonstrate clear advantages in targeting? What is the most critical POI? In which therapeutic areas does it show promise? Is it more beneficial for chronic or acute conditions? Does eTPD primarily target soluble or membrane-bound POIs, or both simultaneously? How crucial is tissue selectivity for safe and effective eTPD drugs? What type of resistance mechanisms will eTPD develop, and is resistance more likely compared to other drug modalities? These questions remain to be answered.
Reference
- Wells, J. A., & Kumru, K. (2024). Extracellular targeted protein degradation: an emerging modality for drug discovery. Nature reviews. Drug discovery, 23(2), 126–140.
- Ahn, G., Banik, S. M., Miller, C. L., Riley, N. M., Cochran, J. R., & Bertozzi, C. R. (2021). LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nature chemical biology, 17(9), 937–946.
- Wu, Y., Lin, B., Lu, Y., Li, L., Deng, K., Zhang, S., Zhang, H., Yang, C., & Zhu, Z. (2023). Aptamer-LYTACs for Targeted Degradation of Extracellular and Membrane Proteins. Angewandte Chemie (International ed. in English), 62(15), e202218106.
- Caianiello, D. F., Zhang, M., Ray, J. D., Howell, R. A., Swartzel, J. C., Branham, E. M. J., Chirkin, E., Sabbasani, V. R., Gong, A. Z., McDonald, D. M., Muthusamy, V., & Spiegel, D. A. (2021). Bifunctional small molecules that mediate the degradation of extracellular proteins. Nature chemical biology, 17(9), 947–953.