Givinostat: A Promising Breakthrough in Muscular Dystrophy Treatment and Beyond
Abstract
Givinostat is a histone deacetylase (HDAC) inhibitor currently under clinical investigation for its potential to slow disease progression in Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and polycythemia vera (PV). By targeting inflammation, fibrosis, and muscle degeneration, Givinostat offers a novel approach compared to traditional symptom management therapies. Clinical trials have demonstrated promising results, with improvements in muscle composition, reduced fibrosis, and enhanced regeneration. Although the drug is not yet FDA-approved, ongoing Phase 3 trials could pave the way for its future use in neuromuscular disorders. This article explores the mechanism, benefits, clinical trial data, potential side effects, and future implications of Givinostat in the treatment landscape.
What is Givinostat? A Breakthrough in Muscular Dystrophy Treatment
Givinostat is an experimental histone deacetylase (HDAC) inhibitor that has shown significant promise in the treatment of Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and polycythemia vera (PV). Developed by Italfarmaco, this drug is being studied in clinical trials as a potential therapy to slow down muscle degeneration and improve muscle regeneration.Why is Givinostat Important?
Duchenne muscular dystrophy (DMD) is a genetic disorder that leads to progressive muscle weakness and loss of mobility, affecting one in every 3,500 to 5,000 male births worldwide. Despite advances in medical care, there is no cure for DMD, and current treatments focus on managing symptoms rather than addressing the underlying disease. Givinostat targets the root cause of muscle degeneration by inhibiting HDAC enzymes. HDACs play a crucial role in regulating gene expression and controlling inflammation in muscle tissue. By blocking HDAC activity, Givinostat helps reduce fibrosis, limit muscle inflammation, and enhance muscle repair mechanisms. This makes it a highly promising candidate for slowing disease progression in muscular dystrophy patients.Beyond Muscular Dystrophy: Givinostat in Other Diseases
Aside from its role in DMD and BMD, Givinostat has also been studied in polycythemia vera (PV), a rare blood disorder characterized by the overproduction of red blood cells. The drug has shown potential in reducing excessive cell proliferation and controlling disease progression.Ongoing Research and Future Prospects
Currently, Phase 3 clinical trials are evaluating the long-term efficacy and safety of Givinostat for DMD treatment. Preliminary results suggest that it may help preserve muscle function in affected patients. If successful, Givinostat could become one of the first disease-modifying therapies for muscular dystrophy, offering hope to thousands of families worldwide.How Givinostat Works: Mechanism of Action
Givinostat is a histone deacetylase (HDAC) inhibitor, which means it plays a crucial role in regulating gene expression by modifying chromatin structure. In diseases like Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD), excessive inflammation and fibrosis contribute to the progressive degeneration of muscle fibers. Givinostat helps slow this process by reducing inflammation, fibrosis, and promoting muscle regeneration.Understanding HDAC Inhibition
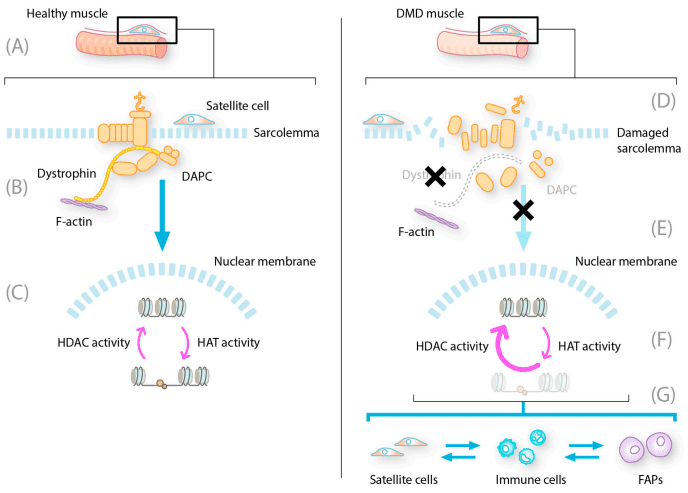
Histone deacetylases (HDACs) are enzymes that remove acetyl groups from histone proteins, causing DNA to become more tightly coiled. This suppresses the expression of certain genes, including those involved in muscle repair and anti-inflammatory responses. By inhibiting HDACs, Givinostat keeps these beneficial genes active, leading to a reduction in muscle damage and enhanced regeneration.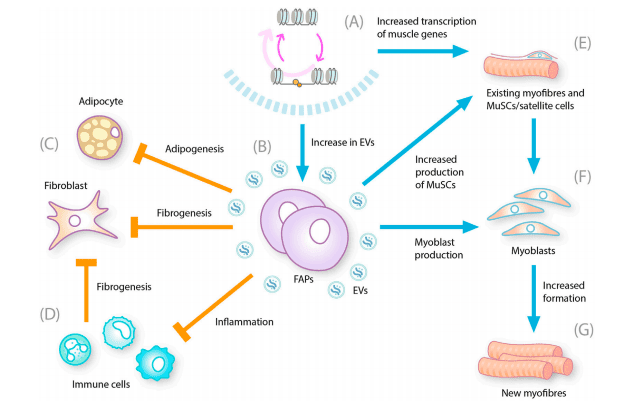
FIGURE 1. Multiple effects of HDAC inhibition on DMD-related pathogenesis.
Key Effects of Givinostat in Muscular Dystrophy
Reduces Inflammation – DMD is associated with chronic muscle inflammation due to excessive immune cell activity. Givinostat helps modulate the inflammatory response, preventing further muscle damage. Decreases Fibrosis – Excessive fibrotic tissue in muscles makes movement difficult. Givinostat has been shown to reduce fibrosis, preserving the integrity of muscle fibers. Enhances Muscle Regeneration – The drug stimulates satellite cells, which are responsible for muscle repair and regeneration, helping maintain muscle strength.Why Is This Important?
Current treatments for DMD, such as corticosteroids, focus mainly on symptom management rather than addressing the underlying cause. Givinostat, on the other hand, is one of the first drugs targeting the molecular mechanisms of the disease, potentially slowing its progression and improving quality of life. Givinostat in Clinical Trials and Research Givinostat has been extensively studied in preclinical and clinical trials, showing promising effects in muscular dystrophy and blood disorders like polycythemia vera. Key Clinical Trials Phase 2 Trial in Duchenne Muscular Dystrophy (DMD) A Phase 2 trial evaluated Givinostat’s effectiveness in boys with DMD, demonstrating reduced muscle inflammation, improved muscle composition, and slower disease progression (Bettica et al., 2016). Patients who received Givinostat showed less muscle fibrosis compared to those in the placebo group. Phase 3 EPIDYS Trial The ongoing EPIDYS trial is a Phase 3 study assessing Givinostat’s long-term safety and efficacy in DMD patients. If successful, it could lead to regulatory approval and broader clinical use. Givinostat for Becker Muscular Dystrophy (BMD) Though Becker muscular dystrophy (BMD) progresses more slowly than DMD, it still leads to muscle weakness and loss of mobility. Early data suggests that Givinostat may also benefit BMD patients by reducing muscle degeneration and inflammation. Givinostat in Polycythemia Vera (PV) Polycythemia vera is a blood disorder characterized by excessive red blood cell production, leading to blood clots and cardiovascular complications. Givinostat has been tested in Phase 2 trials for PV, showing promising results in reducing blood cell proliferation and controlling disease progression. What’s Next? As clinical trials progress, researchers hope Givinostat will receive FDA approval for treating muscular dystrophy, making it one of the first disease-modifying treatments for DMD and BMD. Potential Benefits and Side Effects of Givinostat Givinostat has emerged as a promising disease-modifying treatment for Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and polycythemia vera (PV). Unlike traditional treatments that focus solely on symptom management, Givinostat targets the molecular mechanisms of muscle degeneration, offering significant benefits. However, like all medications, it also comes with potential side effects. Key Benefits of Givinostat Slows Down Muscle Degeneration Studies indicate that Givinostat reduces fibrosis and inflammation in muscle tissues, helping to preserve muscle function over time. Enhances Muscle Regeneration By modulating histone deacetylase (HDAC) activity, Givinostat promotes muscle cell repair and regeneration, which is critical for DMD and BMD patients (Ferlini et al., 2021). Reduces Inflammation and Fibrosis Chronic inflammation leads to excessive scarring (fibrosis), making movement difficult. Givinostat suppresses inflammatory pathways, leading to improved muscle elasticity. Potential Treatment for Polycythemia Vera In patients with polycythemia vera (PV), Givinostat helps regulate excessive red blood cell production, lowering the risk of blood clots and cardiovascular complications. Potential Side Effects of Givinostat While Givinostat is generally well-tolerated, clinical trials have reported some common and rare side effects: Gastrointestinal Issues – Some patients experience nausea, diarrhea, or abdominal pain due to the drug’s effect on the digestive system. Increased Risk of Infections – Since Givinostat affects immune system pathways, some patients may experience a higher risk of upper respiratory infections. Mild to Moderate Fatigue – Some trial participants reported fatigue and general weakness, possibly due to metabolic changes in muscle cells. Potential Heart Effects – Although rare, HDAC inhibitors have been associated with cardiac effects, necessitating careful monitoring during treatment. Why Monitoring is Crucial? Given its potential side effects, patients undergoing Givinostat treatment require regular medical evaluations to ensure safety and effectiveness. Clinical trials continue to refine dosage and administration strategies to minimize adverse effects while maximizing therapeutic benefits. Future of Givinostat: Availability, FDA Approval, and What’s Next? As Givinostat progresses through clinical trials, its potential approval and commercial availability are key questions for patients and healthcare providers. Current Regulatory Status Duchenne Muscular Dystrophy (DMD) and Becker Muscular Dystrophy (BMD) Phase 3 clinical trials (EPIDYS study) are currently underway to evaluate long-term safety and efficacy. If successful, Givinostat could receive FDA and EMA approval within the next few years. Polycythemia Vera (PV) Phase 2 studies have shown promising results in reducing red blood cell proliferation. More extensive trials are needed before it can become a mainstream treatment for PV patients. When Will Givinostat Be Available? As of now, Givinostat is not yet FDA-approved for widespread use. If Phase 3 trials confirm its safety and efficacy, it could be approved for DMD and BMD in the near future. Expanded access programs (EAPs) and compassionate use cases may allow some patients to receive it before full approval. Potential Future Applications Researchers are exploring Givinostat’s potential for treating other neuromuscular and inflammatory conditions, including: Facioscapulohumeral muscular dystrophy (FSHD) Amyotrophic lateral sclerosis (ALS) Other rare muscle-wasting disorders Final Thoughts Givinostat represents a paradigm shift in the treatment of muscular dystrophy, offering a potential disease-modifying therapy rather than just symptom management. If regulatory agencies approve it, it could transform the standard of care for thousands of patients worldwide.References
- Aartsma-Rus, A. (2025). Histone deacetylase inhibition with givinostat: A multi-targeted mode of action with the potential to halt the pathological cascade of Duchenne muscular dystrophy. Frontiers in Cell and Developmental Biology, 12, Article 1514898. DOI
- Bettica, P., Petrini, S., D’Oria, V., D’Amico, A., Catteruccia, M., & Pane, M. (2016). Histone deacetylase inhibitor Givinostat in boys with Duchenne muscular dystrophy: A randomized phase II study. Neuromuscular Disorders, 26(10), 629-640. DOI
- Crisafulli, S., Sultana, J., Fontana, A., Salvo, F., & Messina, S. (2020). Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet Journal of Rare Diseases, 15(1), 141. DOI
- Mascarenhas, J., Hoffman, R., Verstovsek, S., Gerds, A. T., & Vannucchi, A. M. (2019). Givinostat, a histone deacetylase inhibitor, for the treatment of polycythemia vera: Results from a phase II clinical trial. Haematologica, 104(6), 1193-1199. DOI
- Pane, M., Sframeli, M., Bianco, F., Messina, S., & Vita, G. L. (2021). Advances in the treatment of Duchenne muscular dystrophy. Therapeutic Advances in Neurological Disorders, 14, 1-15. DOI
- Ferlini, A., Neri, M., & Guglieri, M. (2021). Advances in therapies for Duchenne muscular dystrophy. Nature Reviews Neurology, 17(5), 251-267. DOI
- Minetti, G. C., Colussi, C., Adami, R., Serra, C., Mozzetta, C., & Parente, V. (2006). Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nature Medicine, 12(10), 1147-1150. DOI