Harnessing the Power of Outer Membrane Vesicles: A New Frontier in Vaccine Development
Abstract
Outer Membrane Vesicles (OMVs) have emerged as a promising platform for the development of novel vaccines, offering several advantages over traditional vaccine strategies. OMVs are naturally released by Gram-negative bacteria and contain immunologically relevant components such as lipopolysaccharides (LPS), proteins, and nucleic acids, which are capable of stimulating both innate and adaptive immune responses. This dual activation mechanism positions OMVs as highly effective candidates for vaccine development, capable of inducing strong humoral and cellular immunity. The future of OMV-based vaccines lies in optimizing their production, enhancing their immunogenicity through genetic engineering, and validating their efficacy in large-scale clinical trials. Despite the challenges, OMVs offer the potential to revolutionize vaccine technology, providing safer and more effective solutions for a wide range of infectious diseases.
Introduction to Outer Membrane Vesicles (OMVs) and Their Role in Vaccine Development
Outer Membrane Vesicles (OMVs) are small, spherical particles that are naturally released by Gram-negative bacteria during growth. These vesicles range from 25 to 250 nm in diameter and are primarily composed of lipids, proteins, and other biomolecules derived from the bacterial outer membrane. OMVs are produced when bacteria shed portions of their outer membrane, which contain a variety of components including surface antigens, lipopolysaccharides (LPS), and proteins, all of which are important for bacterial interaction with the environment and host cells.
In addition to their natural role in bacterial physiology, OMVs have attracted considerable interest as potential vaccine platforms. Their ability to carry diverse biomolecules that are recognized by the immune system makes them highly attractive for vaccine development. OMVs can act as a powerful immunogen by mimicking the bacterial surface, allowing them to induce both humoral and cellular immune responses. This capacity to trigger immune activation is largely due to their surface-exposed antigens and the presence of pathogen-associated molecular patterns (PAMPs), which are recognized by immune receptors such as Toll-like receptors (TLRs).
OMVs represent the envelope of bacteria, with a wide range of surface bacterial antigens in their native conformation and orientation and an optimal size for being uptaken by immune cells. The simultaneous presence of several bacterial antigens, combined with the immunopotentiator effect of the Toll-like receptor (TLR) agonists naturally present on these systems, confer self-adjuvanticity properties to OMVs. These agonists or PAMPs (Pathogen-Associated Molecular Patterns), activators of Pattern Recognition Receptors (PRRs), make OMVs strong drivers of the innate immune response, which functions as the host’s first line of defense. Moreover, several studies have highlighted OMVs’ ability to induce long-lasting humoral and cellular immune responses when used as vaccines. Recently, the potential for inducing a strong immune response together with the simplicity of manufacture, have made OMVs an attractive platform for vaccine development. To this end, genetic manipulations have been introduced in bacteria to further enhance the in vitro blebbing, by the disruption of genes involved in processes including the synthesis of proteins linking the outer membrane and the underlying peptidoglycan layer, envelope structure, and cellular stress. These blebs resulting from the hyperblebbing of genetically modified microorganisms have also been called GMMA (Generalized Modules for Membrane Antigens). The technology has been successfully applied to many pathogens including non-invasive Salmonella, Neisseria meningitidis, and Shigella serotypes, with the most advanced prototype tested up to phase 1 and 2 clinical studies. Several industrial approaches using this and other OMV-based technologies have been used and applied to produce OMVs from several Gram-negative bacteria. Research over the last decade has demonstrated the application of OMVs as powerful, economical, and flexible tools for the delivery of various heterologous vaccine antigens, ranging from bacterial, to viral, parasitic, and even cancer antigens. The antigen per se, or many of the molecules composing the OMV used as delivery system for the heterologous antigen, could act as an effective TLR-activating component, property considered as one of the key drivers for the powerful immune response elicited by OMV.
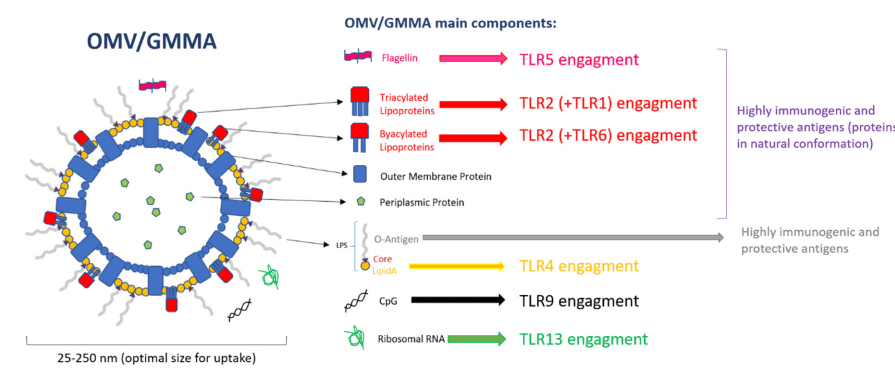
Figure 1. Outer Membrane Vesicle (OMV) components and Toll-like receptor (TLR) engagement.
The growing body of research highlights the potential of OMVs in the development of vaccines for infectious diseases, particularly those caused by bacteria, and their ability to overcome some of the limitations of conventional vaccine formulations. As we delve further into the exploration of OMVs, their application in vaccine technology may become pivotal in the fight against diseases with high unmet medical needs.
The Mechanism of OMVs in Immune Response
Outer Membrane Vesicles (OMVs) play a pivotal role in stimulating the immune system, acting as natural adjuvants that can trigger both innate and adaptive immune responses. These vesicles are derived from the outer membrane of Gram-negative bacteria, carrying a mixture of bioactive components, such as proteins, lipids, and nucleic acids, that are recognized by various receptors of the immune system. OMVs have a remarkable ability to activate immune cells through their diverse composition, making them an appealing candidate for use in vaccine development.
The primary mechanism by which OMVs activate the immune system is through the interaction with Toll-like receptors (TLRs), which are pattern recognition receptors (PRRs) that detect pathogen-associated molecular patterns (PAMPs) present on OMVs. The key components of OMVs, such as lipopolysaccharides (LPS) and surface proteins, are known to interact with specific TLRs, particularly TLR4 and TLR2, to initiate signaling pathways that lead to the production of pro-inflammatory cytokines. These cytokines promote the activation of antigen-presenting cells (APCs), such as dendritic cells and macrophages, which are critical for the initiation of an adaptive immune response.
Once OMVs are internalized by APCs, their antigens are processed and presented on major histocompatibility complex (MHC) molecules, which are then recognized by T cells. This interaction leads to the activation of both humoral and cell-mediated immune responses. The humoral response involves the production of antibodies by B cells, which can neutralize the pathogen, while the cell-mediated response activates cytotoxic T cells that help clear infected cells. The dual action of OMVs in stimulating both arms of the immune system is one of the reasons they are considered highly effective in vaccine formulations.
Moreover, OMVs are capable of mimicking the native structure of the bacteria from which they are derived, ensuring that the immune system recognizes them as part of a pathogen, thereby enhancing the specificity and efficacy of the immune response. This unique characteristic positions OMVs as a promising platform for developing vaccines that are both potent and safe.
TLR Agonists on OMVs and Their Immunological Impact
Outer Membrane Vesicles (OMVs) have gained significant attention as an innovative platform for vaccine development due to their ability to activate the immune system through the presence of Toll-like receptor (TLR) agonists. These agonists are key components that stimulate the innate immune system, triggering signaling pathways that enhance the immune response. The presence of TLR agonists on OMVs allows them to mimic bacterial infections, thus eliciting both humoral and cellular immune responses essential for protective immunity.
TLRs are pattern recognition receptors (PRRs) that are part of the innate immune system. They detect specific molecular patterns called pathogen-associated molecular patterns (PAMPs), which are commonly found on the surface of pathogens like bacteria. In the case of OMVs, the most well-known TLR agonists are lipopolysaccharides (LPS) and lipoproteins, which interact with TLR4 and TLR2, respectively. These interactions trigger a cascade of intracellular signaling events that activate immune cells such as dendritic cells and macrophages, leading to the release of pro-inflammatory cytokines.
Once activated, antigen-presenting cells (APCs) process the antigens present on OMVs and present them on their surface through major histocompatibility complex (MHC) molecules. This process activates T cells, which are crucial for the adaptive immune response. The activation of TLRs by OMVs also boosts the production of interleukins and chemokines, molecules that further enhance the recruitment and activation of additional immune cells, ensuring a robust immune reaction.
OMVs’ ability to present native antigens in their original conformation, alongside their TLR-activating properties, makes them an effective immunogen. The simultaneous stimulation of both the innate and adaptive immune systems offers a multifaceted approach to vaccine development. In fact, OMVs have been shown to provide greater immunogenicity compared to traditional vaccines, potentially reducing the need for additional adjuvants or booster doses.
In conclusion, the TLR agonists expressed on OMVs are critical in promoting an effective immune response, which is essential for the development of OMV-based vaccines. Their ability to stimulate both innate and adaptive immunity positions OMVs as a promising candidate in the evolving field of vaccine technology.
OMVs vs. Traditional Vaccines: A Comparative Analysis
Outer Membrane Vesicles (OMVs) have emerged as a novel platform in vaccine development, offering several advantages over traditional vaccine approaches. OMVs are naturally derived from the outer membrane of Gram-negative bacteria and contain a diverse array of immunologically relevant components such as proteins, lipids, and nucleic acids. These vesicles are particularly advantageous because they can present antigens in their native conformation, facilitating a robust immune response. In comparison to conventional vaccines, OMVs offer unique benefits, although challenges remain in their widespread adoption.
One of the primary advantages of OMVs is their ability to activate both humoral and cellular immune responses. Traditional vaccines often require the use of adjuvants—substances that enhance the body’s immune response to an antigen. OMVs, however, contain inherent Toll-like receptor (TLR) agonists (such as lipopolysaccharides) that stimulate the innate immune system and promote an efficient adaptive immune response without the need for external adjuvants. This dual mechanism of action, where OMVs trigger both antibody production and cytotoxic T cell activation, sets them apart from conventional vaccine strategies, which primarily rely on inducing a humoral response.
Another advantage of OMVs is their ability to mimic the native structure of bacterial pathogens. Since OMVs contain the same antigens found on the surface of the bacteria, they can more effectively stimulate the immune system by presenting these antigens in a biologically relevant manner. Traditional vaccines often utilize inactivated or recombinant forms of these antigens, which may not always present them in the most immunologically active form.
Despite these advantages, there are challenges associated with using OMVs in vaccine development. One such challenge is the scalability of OMV production, as large-scale manufacturing must be optimized for consistency and purity. Furthermore, while OMVs do not typically require adjuvants, they can still exhibit variability in immunogenicity depending on the bacterial strain and environmental conditions used for their production.
In conclusion, OMVs present a promising alternative to traditional vaccine platforms, with the potential to overcome some of the limitations of conventional vaccines. However, further research is needed to optimize their production and ensure their widespread applicability in global vaccination campaigns.
Future Prospects and Conclusion
Outer Membrane Vesicles (OMVs) have demonstrated considerable potential as a next-generation vaccine platform. As vaccine technology continues to evolve, OMVs are increasingly recognized for their ability to trigger both innate and adaptive immune responses, making them a promising alternative to traditional vaccine strategies. The future of OMV-based vaccines looks bright, but several research avenues must be pursued to fully realize their potential in combating infectious diseases.
One of the primary areas of interest is the optimization of OMV production. Although OMVs naturally occur as by-products of bacterial growth, scaling up their production in a controlled and reproducible manner is essential for their use in large-scale vaccine manufacturing. Advances in bioprocessing and genetic engineering of bacterial strains are expected to improve both the yield and purity of OMVs, making them more suitable for use in commercial vaccines. Furthermore, the development of standardized methods for isolating and characterizing OMVs will be crucial in ensuring their safety and efficacy across different bacterial strains and conditions.
In addition to production challenges, vaccine formulation remains a key area of research. While OMVs already contain native bacterial antigens and TLR-activating molecules, researchers are exploring how to enhance their immunogenicity further. One promising approach involves genetic engineering to modify OMVs, allowing them to display a broader array of antigens from multiple pathogens. This would expand the potential applications of OMVs beyond bacterial vaccines, potentially creating multivalent vaccines for viral diseases as well.
The clinical testing of OMV-based vaccines is another critical area that requires attention. Early-stage trials have shown promising results, but larger, more comprehensive clinical studies are needed to assess the long-term safety and efficacy of OMVs in humans. Ensuring that these vaccines produce durable immune responses without significant side effects will be a determining factor in their widespread adoption.
In conclusion, OMVs represent a powerful tool in the development of novel vaccines. With continued advancements in production, formulation, and clinical validation, OMVs have the potential to revolutionize immunization strategies, providing safer and more effective vaccines for a wide range of infectious diseases.
References
- Mancini, F., Rossi, O., Necchi, F., & Micoli, F. (2020). OMV vaccines and the role of TLR agonists in immune response. International Journal of Molecular Sciences, 21(12), 4416.
- Rossi, O., Mancini, F., & Micoli, F. (2020). Outer membrane vesicles: Potential in vaccine development. Vaccine, 38(5), 611-619.
- Clynes, M., & Moxon, E. R. (2018). The use of outer membrane vesicles in vaccine development. Frontiers in Immunology, 9, 2723.
- Cao, H., & Xu, H. (2021). Harnessing outer membrane vesicles for vaccine development: From production to application. Vaccines, 9(4), 399.
- Schell, D. M., & Kuehn, M. J. (2019). Outer membrane vesicles and their role in vaccine development. Frontiers in Immunology, 10, 2701.
- Schwechheimer, C., & Kuehn, M. J. (2015). Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nature Reviews Microbiology, 13(10), 605–619.