How ABT-199 (Venetoclax) is Changing the Treatment Landscape for Blood Cancers
Abstract
ABT-199 (Venetoclax) is a targeted therapy that selectively inhibits the BCL-2 protein, playing a crucial role in promoting apoptosis in cancer cells. Approved for the treatment of hematologic malignancies such as Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin Lymphoma (NHL), venetoclax has shown significant efficacy, particularly in combination therapies. This drug offers a novel approach to cancer treatment by overcoming resistance to traditional chemotherapy. While its clinical impact is profound, challenges related to cost, drug interactions, and side effects remain.Additionally, drug interactions, such as with CYP3A inhibitors and inducers, and risks like tumor lysis syndrome require careful patient management. However, venetoclax’s future in oncology is promising, with ongoing studies expanding its use in solid tumors and refining its application through biomarker-driven treatment. Ultimately, venetoclax represents a significant advancement in personalized cancer therapy, though addressing its affordability and optimizing its combination with other drugs remain critical for maximizing patient outcomes.
Understanding the Mechanism of Action of ABT-199 (Venetoclax)
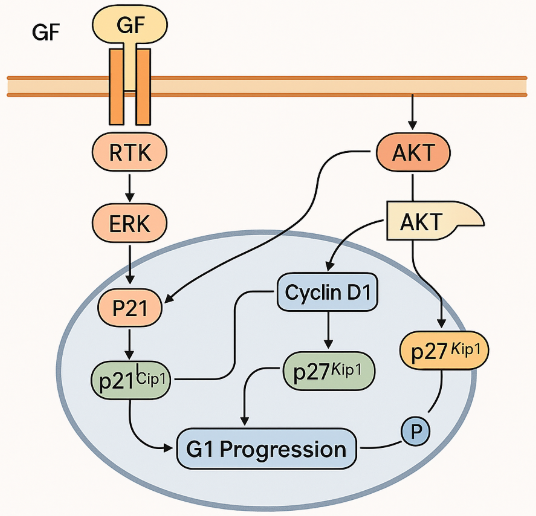
ABT-199 (Venetoclax) is a BCL-2 inhibitor designed to selectively target the BCL-2 protein, which is overexpressed in many cancer cells, particularly in B-cell malignancies such as Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin Lymphoma (NHL). BCL-2 is an anti-apoptotic protein that helps cancer cells evade the normal process of programmed cell death, or apoptosis, thus allowing tumor cells to survive and proliferate. By inhibiting BCL-2, venetoclax restores the apoptotic process, leading to the death of malignant cells.
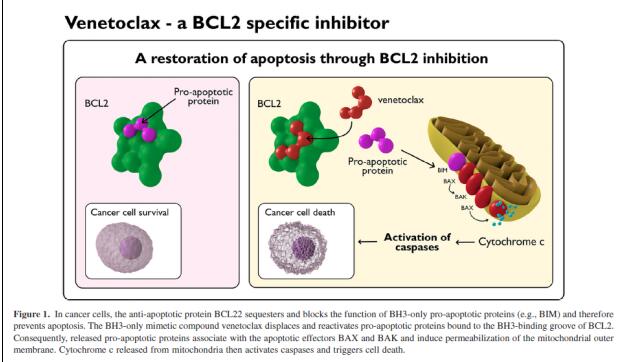
Fig.1 What is Venetoclax/ABT-199?
Venetoclax is considered a form of targeted therapy, which differs from traditional chemotherapy by selectively targeting specific molecules involved in cancer cell survival, rather than indiscriminately killing both cancerous and healthy cells. This targeted approach often results in fewer side effects compared to conventional chemotherapy.
The effectiveness of venetoclax has been demonstrated in clinical trials, showing promising results in treating blood cancers where BCL-2 plays a pivotal role in cancer cell survival. Venetoclax is particularly effective in combination therapies, often used alongside monoclonal antibodies or other chemotherapies, enhancing its therapeutic potential.
Indications for ABT-199 (Venetoclax)
ABT-199 (Venetoclax) has shown significant clinical benefit in treating several types of hematologic malignancies, particularly Chronic Lymphocytic Leukemia (CLL), Non-Hodgkin Lymphoma (NHL), and Acute Myeloid Leukemia (AML). The drug is primarily indicated for patients with CLL, especially those with the 17p deletion, a genetic mutation often associated with poor prognosis and resistance to traditional treatments. Venetoclax has been approved by the FDA for use in combination with other therapies such as rituximab, improving patient outcomes by enhancing the apoptotic response in malignant B-cells.
In addition to CLL, venetoclax has been investigated for other B-cell malignancies, including various subtypes of NHL. Studies have demonstrated its ability to induce remission in patients with relapsed or refractory NHL when used alongside other therapeutic agents, such as obinutuzumab.
Venetoclax is also being explored in Acute Myeloid Leukemia (AML), particularly in combination with hypomethylating agents or chemotherapy. Although its primary use is in hematologic cancers, its potential role in solid tumors is currently under investigation in clinical trials.
ABT-199 (Venetoclax) FDA Approval and Clinical Trials
ABT-199 (Venetoclax), marketed as Venclexta, received FDA approval in 2016 for the treatment of Chronic Lymphocytic Leukemia (CLL) in patients with 17p deletion, a genetic mutation linked to poor outcomes with traditional therapies. The approval was based on the promising results from several clinical trials demonstrating the drug’s ability to induce remission in this hard-to-treat population. Venetoclax is typically used in combination with other therapies like rituximab, which enhances its effectiveness by targeting CD20-positive B-cells.
In addition to its use in CLL, venetoclax has been explored in clinical trials for Non-Hodgkin Lymphoma (NHL) and Acute Myeloid Leukemia (AML). Notably, in NHL, venetoclax has shown positive results when combined with agents like obinutuzumab and chemotherapy, improving patient responses in relapsed or refractory cases. For AML, venetoclax is evaluated in combination with hypomethylating agents or low-dose chemotherapy, showing potential as a treatment for elderly or unfit patients who cannot tolerate intensive chemotherapy.
Venetoclax’s success in clinical trials has led to ongoing research and expansion of its indications in various cancer types, with future trials focused on its use in solid tumors and further combination therapies.
Side Effects and Safety Considerations
Like all cancer therapies, ABT-199 (Venetoclax) comes with a risk of side effects, though its targeted mechanism often leads to fewer systemic side effects compared to traditional chemotherapy. The most common side effects associated with venetoclax include neutropenia (low white blood cell count), anemia (low red blood cell count), nausea, and diarrhea. These are generally manageable and vary depending on the patient’s overall health and the specific regimen used.
One of the most serious potential side effects is tumor lysis syndrome (TLS), which can occur when large numbers of cancer cells are killed off rapidly, leading to the release of cellular contents into the bloodstream. This can result in dangerous metabolic disturbances such as hyperkalemia, hyperuricemia, and acute renal failure. To mitigate this risk, patients are closely monitored during the initial stages of treatment, and precautions like hydration and allopurinol are used to prevent TLS.
Other safety considerations include potential drug interactions, as venetoclax is metabolized by the CYP3A enzyme. Drugs that affect this enzyme can alter venetoclax’s effectiveness or increase the risk of side effects. Furthermore, patients with pre-existing liver conditions or low platelet counts may require dose adjustments.
Despite these risks, venetoclax has proven to be a well-tolerated therapy in most patients, particularly when managed with careful monitoring and supportive care.
Future Directions in ABT-199 (Venetoclax) Research
The clinical success of ABT-199 (Venetoclax) in hematologic malignancies has opened up numerous avenues for future research. Current studies are focusing on expanding its use to solid tumors, such as lung cancer, breast cancer, and pancreatic cancer, where dysregulated apoptosis plays a key role in tumor survival. Combining venetoclax with other targeted therapies, immune checkpoint inhibitors, and chemotherapy regimens is an area of intense investigation, as these combinations may provide synergistic effects and overcome resistance mechanisms commonly seen in advanced cancers.
Moreover, researchers are examining strategies to improve patient selection and identify biomarkers that predict which patients will benefit most from venetoclax therapy. In particular, the role of BCL-2 expression and genetic mutations like 17p deletion in CLL is a key focus for optimizing patient outcomes. Trials are also underway to evaluate the combination of venetoclax with other BCL family inhibitors to block multiple anti-apoptotic pathways in cancer cells.
Lastly, real-world data from ongoing clinical studies and registries will continue to shape our understanding of the long-term benefits and risks of venetoclax, providing insights into dose optimization, treatment duration, and quality of life for patients using this drug in routine clinical practice.
Conclusion: The Future of ABT-199 (Venetoclax) in Oncology
ABT-199 (Venetoclax), a BCL-2 inhibitor, has become a cornerstone in the treatment of hematologic malignancies, particularly Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin Lymphoma (NHL), offering new hope for patients with difficult-to-treat cancers. Its ability to selectively target cancer cells by blocking anti-apoptotic proteins makes it an essential tool in modern oncology. The drug has shown promising results in combination therapies, and ongoing research continues to uncover its potential in treating Acute Myeloid Leukemia (AML) and even solid tumors.
However, challenges remain in terms of cost, accessibility, and side effect management. Efforts to reduce financial barriers and ensure global access to venetoclax are crucial for maximizing its impact. In addition, while its use in hematologic malignancies is already well-established, further studies are necessary to refine its application in solid tumors and explore novel combination therapies.
Looking forward, precision medicine will play a key role in optimizing venetoclax use. Biomarker-driven approaches, such as BCL-2 expression levels and genetic profiles, will likely guide treatment decisions, improving patient outcomes and reducing unnecessary side effects. With ongoing clinical trials and emerging research, venetoclax’s role in oncology is expected to expand, offering a tailored, effective treatment option for more cancer patients in the future.
References
- Ong F, Kim K, Konopleva MY. (2022). Venetoclax resistance: mechanistic insights and future strategies. Cancer Drug Resist, 5(2):380-400. doi: 10.20517/cdr.2021.125. PMID: 35800373; PMCID: PMC9255248.
- Hu M, Li W, Zhang Y, Liang C, Tan J, Wang Y. (2023). Venetoclax in adult acute myeloid leukemia. Biomed Pharmacother, 168:115820. doi: 10.1016/j.biopha.2023.115820. Epub 2023 Nov 6. PMID: 37925935.
- Lasica M, Anderson MA. (2021). Review of Venetoclax in CLL, AML and Multiple Myeloma. J Pers Med, 11(6):463. doi: 10.3390/jpm11060463. PMID: 34073976; PMCID: PMC8225137.
- Garciaz S, Hospital MA, Collette Y, Vey N. (2024). Venetoclax Resistance in Acute Myeloid Leukemia. Cancers (Basel), 16(6):1091. doi: 10.3390/cancers16061091. PMID: 38539426; PMCID: PMC10969100.
- Ucciero A, Pagnoni F, Scotti L, Pisterna A, Barone-Adesi F, Gaidano G, Patriarca A, Lunghi M. (2023). Venetoclax with Hypomethylating Agents in Newly Diagnosed Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis of Survival Data from Real-World Studies. Cancers (Basel), 15(18):4618. doi: 10.3390/cancers15184618. PMID: 37760587; PMCID: PMC10526951.
- Zielonka K, Jamroziak K. (2024). Mechanisms of resistance to venetoclax in hematologic malignancies. Adv Clin Exp Med. doi: 10.17219/acem/181145. Epub ahead of print. PMID: 38439610.
- Mihăilă RG. (2023). Venetoclax in Acute Myeloid Leukemia. Recent Pat Anticancer Drug Discov, 18(1):11-28. doi: 10.2174/1574892817666220429105338. PMID: 35507786.
- Nachmias B, Aumann S, Haran A, Schimmer AD. (2024). Venetoclax resistance in acute myeloid leukaemia-Clinical and biological insights. Br J Haematol, 204(4):1146-1158. doi: 10.1111/bjh.19314. Epub 2024 Jan 31. PMID: 38296617.