Ibrutinib: A Revolutionary Treatment for Blood Cancers
Abstract
Ibrutinib is a medication used to treat various types of blood cancers, including chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), and Waldenstrom’s macroglobulinemia (WM). It belongs to a class of drugs called Bruton’s tyrosine kinase (BTK) inhibitors and works by blocking the action of BTK, a protein that is involved in the growth and survival of cancer cells.
Ibrutinib was first approved by the FDA in 2013 for the treatment of CLL, and has since been approved for use in other types of blood cancers. It is taken orally and is generally well-tolerated, with common side effects including fatigue, diarrhea, nausea, and infections. More serious side effects, such as bleeding, heart problems, and infections, may also occur but are less common.
Clinical studies have shown that ibrutinib can be effective in treating CLL, MCL, and WM, both as a first-line treatment and for patients who have relapsed or become resistant to other therapies. It has also been shown to improve progression-free survival and overall survival in these patients. Ongoing research is exploring the use of ibrutinib in combination with other drugs for even better outcomes, as well as investigating potential biomarkers that may predict response to therapy.
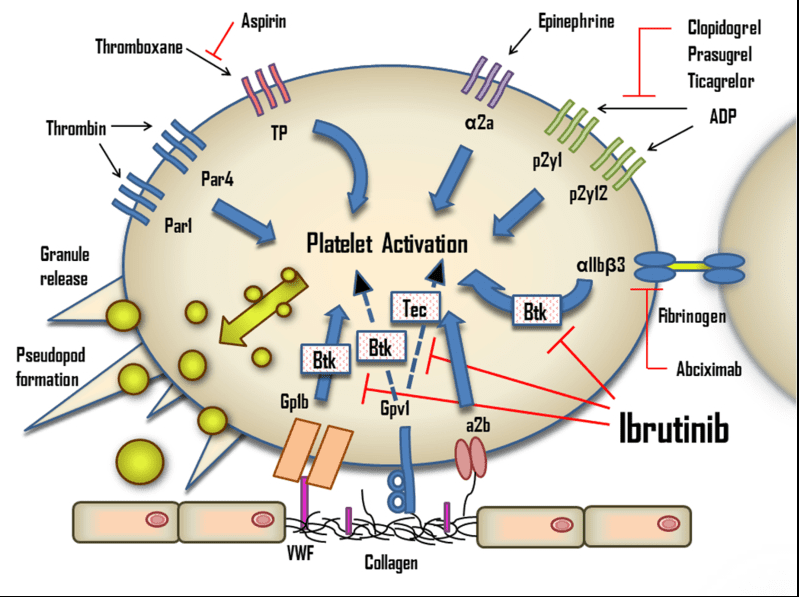
Fig 1. The effects of ibrutinib on platelets. ADP, adenosine diphosphate; Btk, Bruton’s tyrosine kinase; Tec, tyrosine kinase expressed in hepatocellular carcinoma; TP, Thromboxane A2 receptor; VWF, von Willebrand factor.[1]
Chronic lymphocytic leukemia
Chronic lymphocytic leukemia (CLL) is a type of cancer that affects the blood and bone marrow. It is characterized by the accumulation of abnormal lymphocytes, a type of white blood cell, in the blood and lymph nodes. CLL is a slowly progressing disease and is often diagnosed in the early stages, with many patients living for many years without experiencing any symptoms. However, as the disease progresses, patients may experience symptoms such as fatigue, weight loss, and enlarged lymph nodes.
The exact cause of CLL is unknown, but certain risk factors such as age, family history, and exposure to certain chemicals may increase the likelihood of developing the disease. Treatment options for CLL vary depending on the stage and severity of the disease, but may include chemotherapy, immunotherapy, and targeted therapy. Stem cell transplantation may also be considered in certain cases. While there is no cure for CLL, advances in treatment options have greatly improved outcomes for patients, with many living for many years with the disease. Ongoing research is focused on developing new therapies and identifying biomarkers that may help predict response to treatment and improve outcomes for patients with CLL.
Standard first-line treatments for CLL
At present, the standard first-line treatments for CLL include chemotherapy with or without immunotherapy. The most common chemotherapy regimen is a combination of fludarabine, cyclophosphamide, and rituximab (FCR). FCR has been shown to be effective in achieving complete remission in a significant proportion of patients. Other chemotherapy regimens, such as bendamustine and chlorambucil, may also be used, particularly in patients who are not candidates for more intensive therapy.
Immunotherapy with monoclonal antibodies such as rituximab, ofatumumab, and obinutuzumab may be used alone or in combination with chemotherapy. These drugs target specific proteins on the surface of cancer cells and help to activate the immune system to attack and destroy the cancer cells. Overall, the choice of first-line treatment for CLL depends on various factors such as the patient’s age, overall health, and disease stage. Treatment decisions are typically made on an individual basis, taking into account the patient’s unique circumstances and preferences.
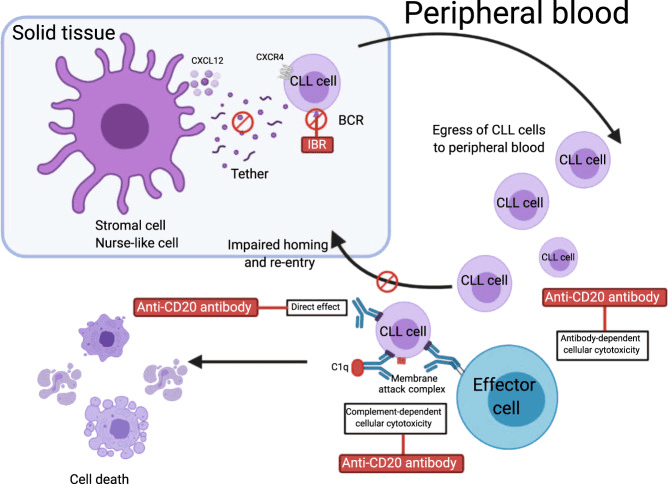
Fig 2. Rationale for ibrutinib combination with monoclonal antibodies.[2]
Ibrutinib (IBR) reduces levels of smCXCR4, leading to enhanced CLL cell egress from the lymph nodes and spleen to the circulation and impaired homing to the nodal compartments. In peripheral blood, CLL cells are exposed to monoclonal antibodies by three major independent mechanisms: (1) antibody-dependent cellular cytotoxicity (ADCC), (2) complement-mediated cytotoxicity, and (3) direct apoptosis. This figure was created with BioRender.com. BCR, B cell receptor.
Ibrutinib is a new treatment option
Ibrutinib is a novel targeted therapy that has revolutionized the treatment of CLL. It works by inhibiting the Bruton’s tyrosine kinase (BTK), a key enzyme involved in the proliferation and survival of CLL cells. By blocking the activity of BTK, ibrutinib induces apoptosis (programmed cell death) of CLL cells, leading to a reduction in tumor burden and an improvement in clinical outcomes.
Ibrutinib was first approved by the US Food and Drug Administration (FDA) in 2013 for the treatment of CLL in patients who have received at least one prior therapy. Since then, it has become a standard of care for the treatment of CLL, both in the relapsed/refractory and the first-line setting. In 2016, ibrutinib was granted accelerated approval by the FDA for the treatment of CLL as a first-line therapy based on the results of the RESONATE-2 clinical trial. The trial showed that ibrutinib was associated with a significant improvement in progression-free survival and overall survival compared to standard chemotherapy in previously untreated CLL patients.
The approval of ibrutinib for the treatment of CLL has significantly improved outcomes for patients with this disease. Its unique mechanism of action, favorable safety profile, and convenient oral dosing make it an attractive treatment option for patients with CLL. Ongoing research is focused on optimizing the use of ibrutinib and developing new combination therapies to further improve outcomes for patients with CLL.
Mechanism of action of ibrutinib
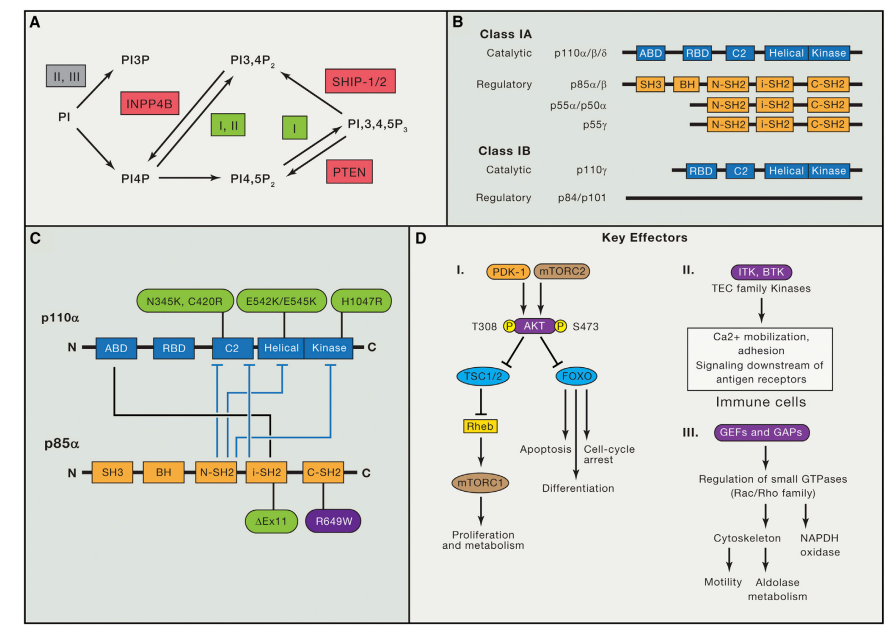
Ibrutinib is a novel targeted therapy that has revolutionized the treatment of chronic lymphocytic leukemia (CLL) and other B-cell malignancies. Its mechanism of action is based on its ability to selectively inhibit the activity of Bruton’s tyrosine kinase (BTK), a key enzyme involved in the signaling pathway of B-cell receptors (BCRs).
BCRs are expressed on the surface of B-cells and are essential for their survival and proliferation. When BCRs are activated by binding to specific antigens, they trigger a signaling cascade that leads to the activation of various downstream enzymes, including BTK. BTK, in turn, activates several other signaling pathways that promote the survival and proliferation of B-cells.
By selectively inhibiting BTK, ibrutinib disrupts the downstream signaling pathways that promote the survival and proliferation of B-cells. This results in the induction of apoptosis (programmed cell death) of CLL cells, leading to a reduction in tumor burden and an improvement in clinical outcomes. Ibrutinib irreversibly binds to a specific site on BTK, called the active site, preventing its phosphorylation and subsequent activation. This inhibitory effect is sustained, leading to prolonged inhibition of BTK activity and sustained apoptosis of CLL cells.
In addition to its effects on CLL cells, ibrutinib has also been shown to have immunomodulatory effects. It can inhibit the activation and proliferation of T-cells and natural killer (NK) cells, leading to an increase in their cytotoxic activity against CLL cells. The mechanism of action of ibrutinib involves the selective inhibition of BTK activity, resulting in the induction of apoptosis of CLL cells and immunomodulatory effects. Its unique mechanism of action has made it an attractive treatment option for patients with CLL and other B-cell malignancies.
Ibrutinib in clinical practice AND Combination therapy
Ibrutinib has rapidly emerged as a standard of care in the treatment of CLL, particularly as a first-line therapy for patients who are not eligible for chemoimmunotherapy. It has demonstrated impressive efficacy in both clinical trials and real-world settings, with high overall response rates and durable remissions. In addition, ibrutinib is generally well-tolerated, with manageable side effects.
One of the major advantages of ibrutinib is its potential for use in combination therapies. Its unique mechanism of action and favorable safety profile make it an attractive partner for other targeted agents, chemotherapy, or immunotherapy. Combination therapies have been shown to improve response rates and deepen remissions, potentially leading to improved outcomes for patients with CLL.
Several clinical trials have investigated the use of ibrutinib in combination with other agents. For example, ibrutinib has been combined with rituximab, obinutuzumab, venetoclax, and idelalisib in clinical trials, with promising results. These combinations have been shown to improve response rates and duration of response, and in some cases, achieve deep and sustained remissions.
However, it is important to note that combination therapies may also increase the risk of adverse events and drug interactions, and may not be appropriate for all patients. Therefore, careful consideration of patient-specific factors and potential risks and benefits is necessary when considering combination therapies with ibrutinib.
Overall, the emergence of ibrutinib as a treatment option for CLL has significantly improved the outlook for patients with this disease. Its unique mechanism of action and potential for combination therapies make it an exciting area of ongoing research and development.
Conclusion
In conclusion, ibrutinib has revolutionized the treatment landscape for patients with CLL. It offers a well-tolerated and effective alternative to traditional chemotherapy and has demonstrated impressive efficacy as both a single-agent and in combination with other therapies. The approval of ibrutinib for CLL has allowed for the treatment of a broader range of patients, including those who are elderly or have comorbidities.
Ibrutinib’s mechanism of action, which targets B-cell receptor signaling, has demonstrated remarkable efficacy as both a single-agent and in combination with other therapies. Ongoing research is investigating the potential of combination therapies, including immunotherapy and chemotherapy, to further improve outcomes for patients. However, it is important to continue monitoring the long-term safety and efficacy of ibrutinib in clinical practice. Additionally, identifying biomarkers and patient-specific factors that may predict response to ibrutinib will be critical for optimizing treatment strategies and improving outcomes.
Overall, ibrutinib represents a significant advancement in the treatment of CLL and holds promise for continued progress in the field of hematologic malignancies.
Reference
Cameron, F., & Sanford, M. (2014). Ibrutinib: first global approval. Drugs, 74, 263-271.
Deeks, E. D. (2017). Ibrutinib: a review in chronic lymphocytic leukaemia. Drugs, 77, 225-236.