Inavolisib: A New Hope in Targeted Therapy for HR+ HER2-Negative Breast Cancer
Abstract
Inavolisib is a PI3K-alpha inhibitor that represents a significant advancement in the treatment of PIK3CA-mutant HR+ HER2-negative breast cancer. By targeting the PI3K-alpha pathway, which is often dysregulated in cancer cells, Inavolisib aims to slow or halt tumor growth while offering a more targeted approach compared to traditional therapies. Clinical trials have shown that Inavolisib, when combined with palbociclib and fulvestrant, can significantly improve progression-free survival and overall response rates in patients with this specific genetic mutation. The drug’s specificity for the PI3K-alpha isoform reduces potential side effects, making it a promising option for patients who have not responded to other forms of endocrine therapy. This blog post explores Inavolisib’s mechanism of action, clinical efficacy, safety profile, and future prospects in personalized breast cancer treatment.
Breast cancer is one of the most common cancers worldwide, and though advances in treatment have improved survival rates, there are still many challenges. One of the most promising recent developments is Inavolisib, a targeted therapy designed to treat hormone receptor-positive (HR+), HER2-negative breast cancer with a PIK3CA mutation. Inavolisib is part of a growing class of drugs that aim to attack cancer more precisely, leading to fewer side effects and better outcomes. This blog explores Inavolisib’s role in modern oncology and its potential to improve the lives of those with advanced breast cancer.
What is Inavolisib?
Inavolisib is a PI3K-alpha inhibitor, a type of drug that specifically targets the PI3K-alpha pathway involved in cell growth and survival. This pathway, when overactive, can fuel the development and spread of cancers, including breast cancer. By blocking this pathway, Inavolisib can slow or stop the growth of tumor cells.
Inavolisib was FDA-approved in 2024 as a treatment option for HR+ HER2-negative breast cancer patients whose tumors harbor a specific mutation in the PIK3CA gene. The PIK3CA mutation is found in a subset of breast cancer cases, and inhibiting the pathway it affects offers a targeted approach to managing the disease.
Fig.1 Inavolisib is a PI3K-alpha inhibitor
Understanding PIK3CA Mutations in Breast Cancer
The PIK3CA gene encodes a protein that is part of the PI3K signaling pathway. This pathway helps regulate important cellular functions like growth, metabolism, and survival. In some cancers, including breast cancer, mutations in PIK3CA can lead to uncontrolled activation of this pathway, allowing cancer cells to proliferate even in the absence of normal growth signals.
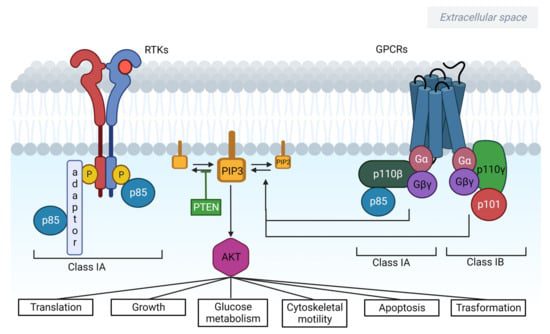
Fig.2 The PI3K/AKT/mTOR pathway is involved in tumorigenesis and cancer progression
In HR+ HER2-negative breast cancer, PIK3CA mutations are particularly prevalent. In fact, these mutations occur in about 40-50% of HR-positive breast cancers, making them a key target for new therapies. By focusing on these specific mutations, therapies like Inavolisib offer a more personalized approach to treatment, attacking cancer cells while leaving normal, healthy cells largely unaffected.
Efficacy of Inavolisib in Clinical Trials
Inavolisib has shown promising results in clinical trials, demonstrating its ability to significantly improve outcomes for patients with PIK3CA-mutant HR+ HER2-negative breast cancer. When combined with palbociclib (a CDK4/6 inhibitor) and fulvestrant (an estrogen receptor antagonist), Inavolisib has been shown to extend progression-free survival (PFS) and improve overall response rates compared to traditional therapies.
In trials, patients receiving this combination treatment have experienced significant tumor shrinkage and a reduced risk of cancer progression, offering new hope to those whose cancer had not responded to other forms of endocrine therapy.
Inavolisib vs Other PI3K Inhibitors
While Inavolisib is part of a class of drugs called PI3K inhibitors, it distinguishes itself through its targeting of the PI3K-alpha isoform, which is specifically implicated in breast cancer. Other PI3K inhibitors, like Alpelisib and Idelalisib, are used for different types of cancers but can target other isoforms of the PI3K pathway, such as PI3K-beta, delta, and gamma. This broader targeting can sometimes lead to greater side effects, making Inavolisib’s specificity for PI3K-alpha a significant advantage.
Compared to Alpelisib, which is commonly used for similar indications, Inavolisib may offer fewer off-target effects, which means a potentially better safety profile and less toxicity for patients. Clinical trials have shown that Inavolisib, in combination with other therapies, may offer superior efficacy for patients with specific PIK3CA mutations, though ongoing studies will continue to refine our understanding of how it stacks up against other therapies in real-world settings.
Side Effects and Safety Profile
As with any cancer treatment, Inavolisib does come with the possibility of side effects. The most commonly reported issues include:
- Fatigue
- Nausea
- Diarrhea
- Elevated liver enzymes (which may indicate liver stress)
These side effects are generally manageable and can often be controlled with supportive treatments or dosage adjustments. However, Inavolisib also carries the risk of more serious side effects, including:
- Liver toxicity
- Pulmonary toxicity (inflammation of the lungs)
- Cardiovascular issues (though rare)
Regular monitoring of liver function and other vital signs during treatment is essential to ensure the safety of patients on Inavolisib. It’s important to discuss any potential side effects with a healthcare provider before starting treatment to develop a management plan tailored to individual needs.
Who Should Consider Inavolisib?
Inavolisib is specifically designed for patients who have PIK3CA-mutant HR+ HER2-negative breast cancer. If a patient’s breast cancer tests positive for the PIK3CA mutation, they may be eligible for Inavolisib treatment, especially if their cancer has progressed despite previous endocrine therapy.
Genetic testing is crucial in identifying whether a patient’s tumor carries the PIK3CA mutation. This testing can guide treatment decisions and ensure that patients receive the most effective therapy available. Inavolisib is often used in combination with other drugs, such as palbociclib and fulvestrant, to enhance its therapeutic benefits.
The Future of Inavolisib and Breast Cancer Treatment
While Inavolisib has already shown great promise, the future of breast cancer treatment is constantly evolving. Ongoing clinical trials are exploring new ways to combine Inavolisib with other therapies, such as immunotherapies, to improve its efficacy further.
There is also interest in using Inavolisib for other cancers with PIK3CA mutations, broadening its therapeutic potential. Researchers are studying whether Inavolisib could benefit patients with other types of tumors that also rely on the PI3K-alpha pathway for growth.
As new treatments emerge, Inavolisib represents an exciting step forward in the era of personalized medicine, offering patients with advanced breast cancer new hope and better chances of long-term disease control.
Conclusion
Inavolisib is a promising treatment for patients with PIK3CA-mutant HR+ HER2-negative breast cancer, providing a new option for those whose cancer has become resistant to traditional therapies. With its targeted mechanism of action, Inavolisib not only offers improved efficacy but also fewer side effects compared to some other cancer treatments.
As breast cancer research continues to evolve, therapies like Inavolisib stand at the forefront of the fight against cancer, offering hope and better survival prospects for patients around the world. If you or a loved one have been diagnosed with PIK3CA-mutant breast cancer, it may be worth discussing Inavolisib with your oncologist to see if it is the right treatment option.
References
- Turner NC, Im SA, Saura C, et al. (2024). Inavolisib-Based Therapy in PIK3CA-Mutated Advanced Breast Cancer. N Engl J Med, 391(17), 1584-1596. DOI
- Jhaveri KL, Accordino MK, Bedard PL, et al. (2024). Phase I/Ib Trial of Inavolisib Plus Palbociclib and Endocrine Therapy for PIK3CA-Mutated, Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced or Metastatic Breast Cancer. J Clin Oncol, 42(33), 3947-3956. PDF
- Salphati L, Pang J, Plise EG, et al. (2024). Preclinical assessment of the PI3Kα selective inhibitor inavolisib and prediction of its pharmacokinetics and efficacious dose in human. Xenobiotica, 54(10), 808-820. DOI
- Zhang J. (2025). FDA Approves Inavolisib Combo for PIK3CA-Mutated, HR+ Breast Cancer. Curr Med Chem. DOI