Incretin Mimetics: Promising Therapeutic Agents for Neuroinflammation and Neurodegenerative Diseases
Abstract
Neuroinflammation is a key contributor to the progression of neurodegenerative diseases and brain injuries, such as Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and traumatic brain injury (TBI). Despite significant advances in understanding these conditions, there remains a lack of FDA-approved drugs to effectively mitigate their progression. A recent genome-wide association study (GWAS) has identified inflammation-related pathways as pivotal drivers of risk in AD, highlighting the potential for repurposing FDA-approved drugs to target neuroinflammation. Incretin mimetics, originally developed for the treatment of Type 2 diabetes mellitus (T2DM), have emerged as promising candidates due to their potent anti-inflammatory, neuroprotective, and neurotrophic effects. Preclinical studies have demonstrated their efficacy in various animal models of stroke, PD, AD, glaucoma, and TBI, while early-stage clinical trials in PD have yielded positive results. This review provides an overview of incretin-based therapies, focusing on their dual role in blood glucose regulation and neuroprotection. It also discusses recent advancements in incretin mimetic drugs, including GLP-1 receptor (GLP-1R) and GIP receptor (GIPR) agonists, as well as multi-receptor agonists like tirzepatide, and their potential for treating neurodegenerative diseases. Given their established safety profiles, incretin mimetics hold considerable promise for repurposing in the treatment of neurological disorders, offering new hope for patients with chronic and acute brain injuries.
Incretin Overview
Neuroinflammation plays a pivotal role in the progression and recovery of neurodegenerative diseases and brain injuries. Unfortunately, there is a significant gap in FDA-approved medications aimed at halting or mitigating the progression of chronic brain disorders such as Parkinson’s Disease (PD) and Alzheimer’s Disease (AD), as well as acute brain injuries like traumatic brain injury (TBI). A recent genome-wide association study (GWAS) offers new genetic insights into AD and related dementias, highlighting inflammation-related pathways as crucial drivers of risk. The repurposing of FDA-approved drugs to target inflammation in central nervous system (CNS) diseases and injuries could be highly beneficial, as their safety profiles in humans are already well-established. Among these, incretin mimetics, originally developed for the treatment of Type 2 diabetes mellitus (T2DM), have emerged as a promising class of drugs for repurposing in the treatment of neurological disorders. Preclinical studies in animal models of stroke, PD, AD, glaucoma, and TBI suggest the potential efficacy of incretin mimetics as therapeutic agents. In addition, clinical trials focusing on CNS diseases are either ongoing or have been completed, with early-stage trials in PD showing positive results. The multifaceted signaling effects of incretin mimetics in various cell types, coupled with their potent anti-inflammatory properties, neurotrophic, and neuroprotective effects, make them ideal candidates for further investigation in clinical trials targeting neurodegenerative diseases.
This review provides an overview of incretin mimetic drugs—both FDA-approved and those in clinical and preclinical stages of development—emphasizing their potent anti-inflammatory potential and the promise they hold as candidates for repurposing in neurodegenerative diseases.
Incretins – Focus on the Endocrine System
The incretin signaling system, which includes the gut-derived metabolic peptides glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP), is essential for blood glucose regulation following food intake. These peptides stimulate insulin release and are secreted by enteroendocrine L and K cells in the small intestine, respectively. GLP-1 and GIP act on their respective receptors, GLP-1R and GIPR, both of which are G protein-coupled receptors (GPCRs). These receptors mediate a range of actions in the endocrine system, including insulin secretion, β-cell survival, triacylglycerol uptake by adipose tissue, and bone resorption. The actions of GLP-1 and GIP complement and oppose those of glucagon (Gcg), a hormone that raises blood glucose during fasting. Once released, these incretin hormones are quickly degraded by the enzyme dipeptidyl peptidase-4 (DPP-IV), which cleaves them to shorten their action and thereby regulates their physiological effects.
Research has shown that T2DM patients exhibit reduced incretin effects, prompting further investigations into GLP-1 and GIP as potential therapies. While both peptides are insulinotropic in healthy individuals, GLP-1 maintains its insulinotropic effects in T2DM, making GLP-1 receptor agonists a focus for drug development.
FDA Approval of Incretin-Based Therapies
Various GLP-1 analogs have been developed to enhance the half-life of GLP-1 and extend the benefits of GLP-1R signaling. One of the earliest breakthroughs in this field came in 1992 with the discovery of exendin-4, an endogenous GLP-1 analog from the venom of the Gila monster (Heloderma suspectum). Exendin-4 is resistant to DPP-IV cleavage and has a prolonged half-life compared to GLP-1, making it suitable for therapeutic use. In 2005, exendin-4, marketed as Exenatide, became the first FDA-approved GLP-1R agonist for T2DM treatment. Since then, other GLP-1 analogs, including liraglutide, semaglutide, lixisenatide, and dulaglutide, have been approved. Additionally, DPP-IV inhibitors have been FDA-approved to increase the levels of endogenous incretins, offering another treatment option for T2DM.
Multi-Agonism
More recent research has focused on dual and multi-receptor agonists, which target GLP-1R, GIPR, and the glucagon receptor (GcgR) to treat metabolic disorders. The development of dual agonists like the GLP-1R/GcgR agonist for metabolic disorders in rodents in 2009 paved the way for further studies. The 2013 development of “twincretin,” a dual GLP-1R/GIPR agonist, showed efficacy in reducing HbA1c in both animal models and humans with T2DM. One of the most advanced agents, Tirzepatide, a dual GLP-1R/GIPR agonist, has demonstrated superior efficacy in preclinical and clinical trials, reducing HbA1c, blood glucose levels, and promoting weight loss. Tirzepatide (Mounjaro™) was FDA-approved in 2022. Additionally, triagonists, which target GLP-1, GIP, and Gcg receptors, have shown promising results in preclinical trials, suggesting they could offer enhanced benefits over dual agonists.
In clinical trials, SAR441255, a GLP-1R/GIPR/GcgR triagonist, demonstrated superior efficacy in rodent models, improving blood glucose regulation and reducing body weight. However, business decisions may hinder its further clinical development. Another promising triagonist, HM15211, is undergoing human trials, with a focus on obesity and non-alcoholic steatohepatitis (NASH). The future of incretin-based therapies lies in the growing body of evidence supporting multi-agonism, which is likely to dominate the treatment landscape for diabetes and obesity in the coming decade.
Insulin Resistance and Introducing Incretins in the CNS
In healthy individuals, the balance between GLP-1, GIP, and Gcg signaling is carefully maintained. Disruption of this balance leads to elevated blood glucose levels and the development of insulin resistance (IR), a hallmark of T2DM and obesity. IR can impair insulin signaling in the brain, contributing to neurodegeneration, as seen in diseases like AD and PD. Interestingly, brain IR can occur independently of peripheral IR, suggesting that the central nervous system (CNS) may experience its own form of IR that contributes to neurodegenerative disease progression. Additionally, T2DM patients who suffer a TBI have higher mortality rates, with blood glucose dysregulation after injury further complicating recovery.
Incretin receptors, particularly GLP-1R and GIPR, are widely expressed in the brain and are involved in regulating feeding behaviors, glucose homeostasis, and cognitive functions. GLP-1R signaling in the hippocampus and hypothalamus has been linked to enhanced memory function and appetite suppression. Animal studies have shown that GLP-1R stimulation protects neurons from excitotoxicity and promotes neuronal growth and differentiation. Moreover, GLP-1R-deficient mice exhibit cognitive deficits, further emphasizing the importance of GLP-1R signaling in neuroprotection.
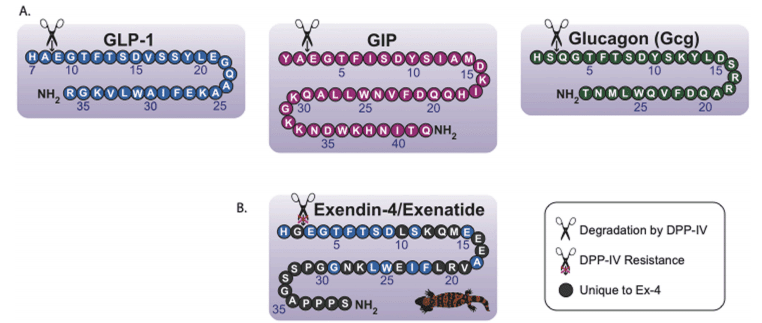
Fig. 1. The endogenous secretins have similar amino acid sequences and structures, most notably their favorable cleavage sites for DPP-IV.
As incretin mimetics are investigated for their potential neuroprotective effects, one key challenge remains their ability to cross the blood-brain barrier (BBB). While GLP-1 and other incretin peptides are large molecules with limited BBB penetration, several studies have demonstrated that specific agonists, such as exendin-4, can cross the BBB in small amounts, exerting pharmacological effects on brain regions involved in memory and glucose regulation. Researchers are exploring modifications to incretin peptides to enhance BBB penetration, as exemplified by the development of PEGylated GLP-1R agonists.
Conclusion
Incretin mimetics, particularly those targeting GLP-1R and GIPR, show significant promise as therapies for neurodegenerative diseases, leveraging their anti-inflammatory, neuroprotective, and neurotrophic properties. With growing evidence from preclinical and clinical studies, these drugs could potentially revolutionize the treatment of both chronic and acute brain disorders. As the development of multi-agonists progresses, the future of incretin-based therapies may expand beyond metabolic diseases to include neurodegenerative conditions, offering hope for patients with limited treatment options.
References
Malhotra, K., Katsanos, A. H., Lambadiari, V., Goyal, N., Palaiodimou, L., Kosmidou, M., Krogias, C., Alexandrov, A. V., Tsivgoulis, G. (2020). GLP-1 receptor agonists in diabetes for stroke prevention: A systematic review and meta-analysis. Journal of Neurology, 267(7), 2117–2122. https://doi.org/10.1007/s00415-020-09877-3
Glotfelty, E. J., Olson, L., Karlsson, T. E., Li, Y., & Greig, N. H. (2020). Glucagon-like peptide-1 (GLP-1)-based receptor agonists as a treatment for Parkinson’s disease. Expert Opinion on Investigational Drugs, 29(6), 595–602. https://doi.org/10.1080/13543784.2020.1794021
Boccardi, V., Murasecco, I., & Mecocci, P. (2019). Diabetes drugs in the fight against Alzheimer’s disease. Ageing Research Reviews, 54, 100936. https://doi.org/10.1016/j.arr.2019.100936
Cui, Q. N., Stein, L. M., Fortin, S. M., & Hayes, M. R. (2022). The role of glia in the physiology and pharmacology of glucagon-like peptide-1: Implications for obesity, diabetes, neurodegeneration, and glaucoma. British Journal of Pharmacology, 179(4), 715–726. https://doi.org/10.1111/bph.16014
Asmar, M., Simonsen, L., Madsbad, S., Stallknecht, B., Holst, J. J., & Bulow, J. (2010). Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes, 59(9), 2160–2163. https://doi.org/10.2337/db10-0270
Hui, H., Farilla, L., Merkel, P., & Perfetti, R. (2002). The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects. European Journal of Endocrinology, 146(6), 863–869. https://doi.org/10.1530/eje.0.1460863
Pontiroli, A. E., Calderara, A., Perfetti, M. G., & Bareggi, S. R. (1993). Pharmacokinetics of intranasal, intramuscular, and intravenous glucagon in healthy subjects and diabetic patients. European Journal of Clinical Pharmacology, 45(6), 555–558. https://doi.org/10.1007/BF00974865
Kopp, K. O., Glotfelty, E. J., Li, Y., & Greig, N. H. (2022). Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment. Pharmacological Research, 182, 106550. https://doi.org/10.1016/j.phrs.2022.106550




