Lenalidomide Used in Cancer Therapies Based on Targeted Protein Degradation
Abstract
Lenalidomide stands as a remarkable example of targeted protein degradation in cancer therapy. This drug, originally developed as an immunomodulatory agent, has garnered significant attention for its ability to harness the cellular machinery for protein degradation, specifically in the context of oncology.
Introduction
Lenalidomide stands as a remarkable example of targeted protein degradation in cancer therapy. This drug, originally developed as an immunomodulatory agent, has garnered significant attention for its ability to harness the cellular machinery for protein degradation, specifically in the context of oncology. Lenalidomide exerts its effects by redirecting targeted proteins towards proteasomal degradation, thereby impacting critical pathways involved in tumor progression and immune responses. One of the key features of lenalidomide is its influence on specific target proteins, including IKZF1 and IKZF3, which play pivotal roles in multiple myeloma and other hematologic malignancies[1]. By inducing the degradation of these proteins, lenalidomide effectively disrupts the cancer cells’ survival mechanisms, ultimately leading to their demise. The clinical applications of lenalidomide have expanded well beyond its initial indications. It has become a cornerstone in multiple myeloma treatment and holds promise for other cancers. The success of lenalidomide underscores the potential of targeted protein degradation as a novel and effective approach in cancer therapy, opening doors for further research and the development of similar compounds that can manipulate protein degradation pathways for therapeutic benefit.
Mechanism of Action of Lenalidomide
Lenalidomide, a potent immunomodulatory drug, has gained significant attention and clinical use in the treatment of various hematological malignancies. Its mechanism of action encompasses several distinct yet interconnected pathways, making it a versatile therapeutic agent. In this section, we will delve into the intricacies of lenalidomide’s mechanisms, focusing on three key aspects: immunomodulation, inhibition of angiogenesis, and enhancement of T-cell and natural killer cell activity.
A. Immunomodulation
Lenalidomide’s immunomodulatory effects lie at the heart of its therapeutic potential. It exerts its influence on the immune system through multiple avenues, primarily by enhancing the activity of immune effector cells and suppressing the activity of immunosuppressive elements.
One of the pivotal targets of lenalidomide is the modulation of T-cell function[2]. It has been shown to increase the proliferation and cytokine production of T-cells, particularly CD4+ and CD8+ T-cells. This enhanced T-cell activity plays a crucial role in eliminating cancer cells by directly targeting them or by facilitating antibody-dependent cellular cytotoxicity (ADCC). Furthermore, lenalidomide can augment the cytotoxic activity of natural killer (NK) cells, making them more effective at recognizing and destroying cancer cells. By promoting these effector cells, lenalidomide contributes to a robust immune response against malignancies.
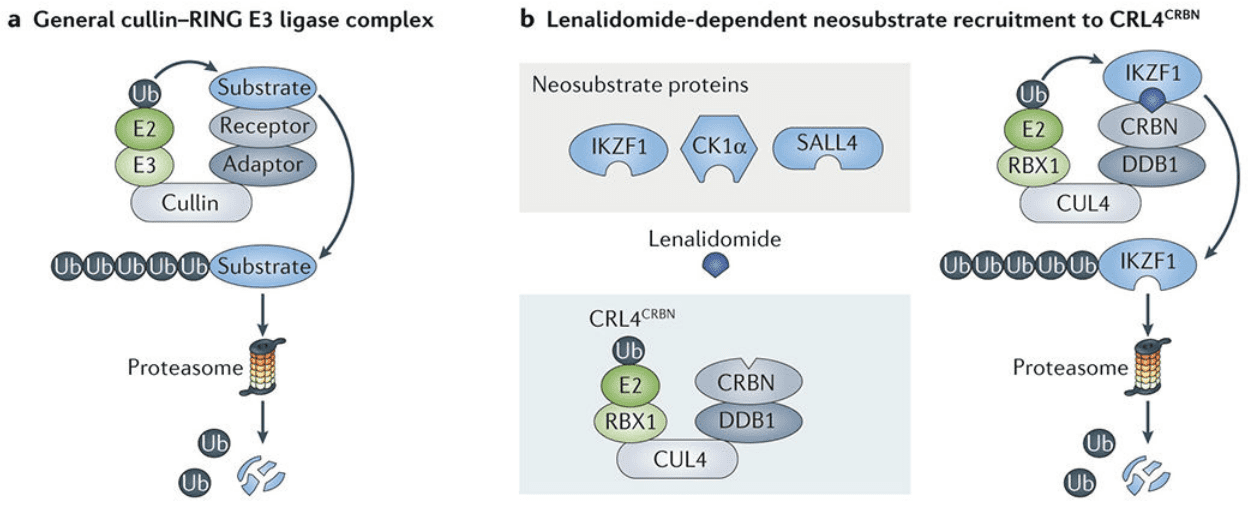
Figure 1 Mechanisms of targeted protein degradation.[2]
Additionally, lenalidomide exerts an inhibitory effect on regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs). Tregs play a role in suppressing immune responses, and lenalidomide’s impact on them helps to counteract this inhibition. MDSCs are a group of immature myeloid cells that can suppress the immune system, and lenalidomide reduces their numbers and inhibitory function. This dual effect on Tregs and MDSCs further promotes an immune environment that is unfavorable for cancer cells, enhancing the anti-tumor immune response.
B. Inhibition of Angiogenesis
Angiogenesis, the process of new blood vessel formation, is a critical aspect of tumor growth and metastasis. Tumors depend on the development of a vascular network to supply them with nutrients and oxygen. Lenalidomide plays a role in inhibiting angiogenesis through several mechanisms.
One of the key targets in this process is vascular endothelial growth factor (VEGF). Lenalidomide reduces the production of VEGF by cancer cells, thereby limiting the signals for new blood vessel formation. This inhibition of VEGF production is complemented by lenalidomide’s ability to reduce the expression of pro-angiogenic factors. By impeding the angiogenic signals, lenalidomide interferes with the formation of new blood vessels within the tumor microenvironment.
Furthermore, lenalidomide affects the tumor-associated endothelial cells, which are responsible for constructing blood vessels within the tumor. It can lead to the direct apoptosis of these endothelial cells, thereby impairing the development of tumor blood vessels. These combined effects on angiogenesis make lenalidomide an effective tool in limiting the blood supply to tumors, ultimately leading to tumor regression.
C. Enhanced T-Cell and Natural Killer Cell Activity
In addition to its immunomodulatory effects, lenalidomide enhances the function of T-cells and natural killer (NK) cells, making them more potent defenders against cancer cells.
- cell activation is a crucial component of the immune response against malignancies. Lenalidomide stimulates the proliferation and cytokine production of CD4+ and CD8+ T-cells. CD4+ T-cells help coordinate immune responses and play a role in enhancing the activity of other immune cells. CD8+ T-cells, on the other hand, are cytotoxic T-cells that can directly target and kill cancer cells. Lenalidomide’s promotion of T-cell activity contributes to a more robust anti-tumor immune response.
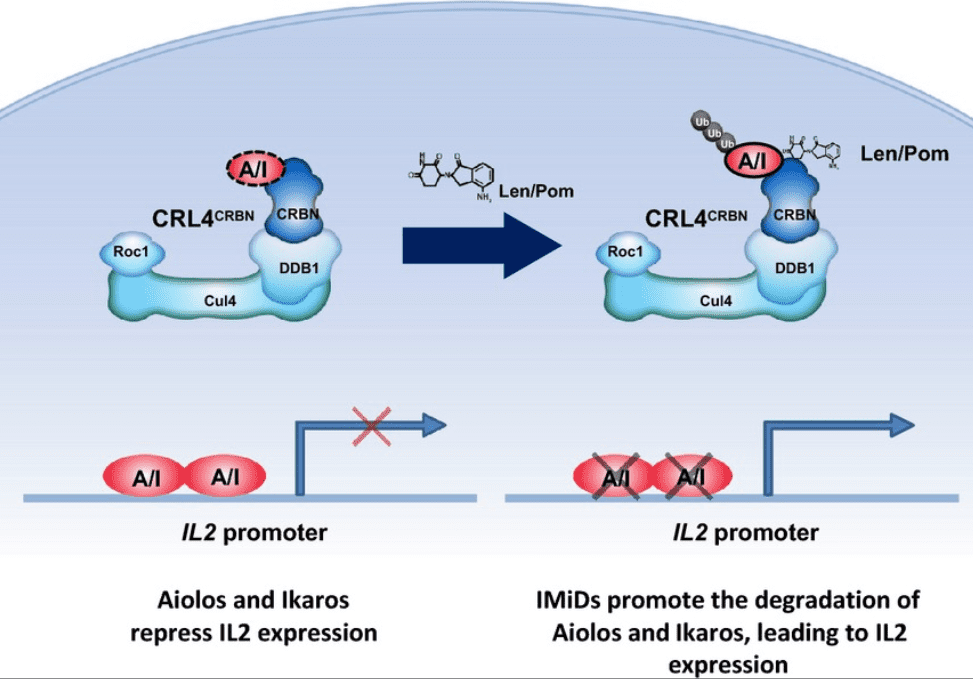
Figure 2 Model of lenalidomide and pomalidomide Co-stimulation of T cells via Degradation of Aiolos and Ikaros[6]
Natural killer (NK) cells are another essential component of the immune system’s defense against cancer. Lenalidomide enhances the cytotoxic activity of NK cells, making them more effective at recognizing and destroying cancer cells. This effect is particularly valuable in combating hematological malignancies, where NK cells play a crucial role in tumor surveillance.
Overall, lenalidomide’s ability to augment the activity of T-cells and NK cells contributes to a more effective immune response against cancer cells. By enhancing the cytotoxic potential of these immune effector cells, lenalidomide strengthens the body’s ability to eliminate malignant cells. Lenalidomide’s mechanism of action is a multi-faceted approach that encompasses immunomodulation, inhibition of angiogenesis, and enhancement of T-cell and natural killer cell activity. These interconnected pathways work in harmony to create an immune microenvironment that is hostile to cancer cells and inhibits their growth. Understanding the complex interplay of these mechanisms is essential for appreciating the therapeutic value of lenalidomide in the treatment of hematological malignancies and other conditions.
Clinical Applications of Lenalidomide
Lenalidomide, a versatile immunomodulatory drug with multifaceted mechanisms of action, has demonstrated remarkable efficacy in the treatment of various hematological malignancies. Its clinical applications span a range of conditions, with particularly notable success in the following areas:
A. Multiple Myeloma
Multiple myeloma, a hematological cancer characterized by the uncontrolled proliferation of plasma cells within the bone marrow, is a prime target for lenalidomide therapy. Lenalidomide has become a cornerstone in the treatment of multiple myeloma, both in newly diagnosed patients and those with relapsed or refractory disease.
In newly diagnosed multiple myeloma, lenalidomide is often used in combination with other agents[3], such as dexamethasone and proteasome inhibitors like bortezomib or carfilzomib. This combination therapy results in high response rates and extended progression-free survival. The immunomodulatory properties of lenalidomide play a pivotal role in enhancing the anti-myeloma immune response, while its inhibition of angiogenesis helps curtail the growth of the tumor microenvironment.
For patients with relapsed or refractory multiple myeloma, lenalidomide has demonstrated its effectiveness as a single agent or in combination with other treatments. It offers a valuable therapeutic option, often providing disease control and symptom relief.
B. Myelodysplastic Syndromes
Lenalidomide has also found significant clinical utility in the treatment of myelodysplastic syndromes (MDS), a group of hematological disorders characterized by dysfunctional blood cell production[4]. Specifically, lenalidomide is approved for the management of transfusion-dependent MDS with a deletion 5q cytogenetic abnormality.
In this context, lenalidomide showcases its ability to promote erythropoiesis, the production of red blood cells. Patients with del(5q) MDS typically suffer from anemia due to a loss of genetic material on chromosome 5. Lenalidomide’s targeted approach in this specific subset of MDS patients leads to an improvement in hemoglobin levels and a reduction in the need for blood transfusions.
C. Other Hematological Malignancies
Beyond multiple myeloma and MDS, lenalidomide is being explored and utilized in the management of various other hematological malignancies, further highlighting its versatility[5]. Clinical studies are ongoing to assess its efficacy in conditions such as chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), and myelofibrosis.
In CLL, lenalidomide has demonstrated promise, especially in combination with other agents, offering a potential therapeutic option for patients who have relapsed or refractory disease. Similarly, in NHL, lenalidomide is being investigated for its potential in enhancing immune responses against lymphoma cells.
Lenalidomide’s role in myelofibrosis, a condition characterized by bone marrow scarring and impaired blood cell production, is an area of active research. Preliminary studies have shown some beneficial effects in terms of symptom control and reduction of bone marrow fibrosis.
Lenalidomide’s clinical applications extend to a spectrum of hematological malignancies, making it a versatile and valuable therapeutic option in the field of oncology. Its use in multiple myeloma, MDS with del(5q), and ongoing investigations in other conditions showcase its potential to improve patient outcomes and provide much-needed hope to those battling these complex diseases. As research continues, lenalidomide’s role in treating hematological malignancies may expand further, offering new avenues for patient care and treatment options.
Lessons Learned
A. Efficacy and Safety of Lenalidomide in Clinical Settings
Lenalidomide’s journey from a promising compound to a cornerstone in cancer therapy has provided valuable insights into its efficacy and safety in clinical settings. Its application in treating various hematological malignancies, particularly multiple myeloma and myelodysplastic syndromes, has yielded important lessons.
One of the key lessons learned is the substantial clinical efficacy of lenalidomide. In the treatment of multiple myeloma, it has shown the ability to induce high response rates, prolong progression-free survival, and improve overall survival when used in combination with other agents. The drug’s immunomodulatory properties and inhibition of angiogenesis have proven to be powerful tools in the fight against cancer.
Moreover, lenalidomide’s safety profile, while not devoid of side effects, has been manageable in most patients. Common adverse events include myelosuppression, particularly neutropenia and thrombocytopenia, as well as non-hematological side effects such as fatigue and gastrointestinal symptoms. Effective strategies for monitoring and managing these side effects have been developed, contributing to its safety in clinical practice.
B. Insights into Targeted Protein Degradation as a Cancer Therapy Strategy
Lenalidomide’s success has shed light on the broader strategy of targeted protein degradation as a novel approach to cancer therapy. The drug’s mode of action involves the degradation of specific proteins, making it a pioneering example in this field.
This approach, known as targeted protein degradation, has the advantage of precision. Instead of simply inhibiting the activity of a protein, it promotes its degradation, leading to a more sustained and potent effect. The lessons learned from lenalidomide’s use have encouraged researchers to explore other agents and strategies for targeted protein degradation.
The concept of targeted protein degradation has far-reaching implications for cancer therapy. It has the potential to address drug resistance, as it can target proteins that contribute to resistance mechanisms. Moreover, it offers opportunities for personalized medicine, as it can be tailored to the specific proteins involved in a patient’s cancer.
C. Future Directions and Potential Improvements
As the clinical experience with lenalidomide continues to evolve, it provides a springboard for exploring future directions and potential improvements in cancer therapy. Several avenues of research and development are being pursued to build upon the lessons learned from lenalidomide’s use.
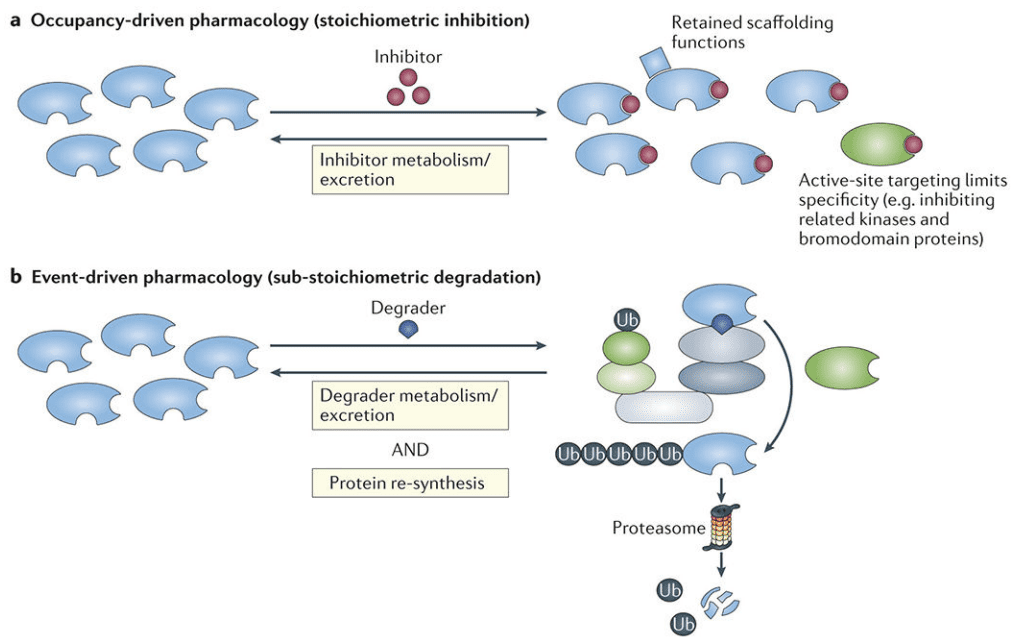
Figure 3 Event-driven versus occupancy-driven pharmacology[2]
- Combination Therapies: The success of lenalidomide in combination with other agents has inspired investigations into more sophisticated combination therapies. Identifying synergistic drug combinations can improve efficacy while potentially reducing side effects.
- Expansion to Solid Tumors: While lenalidomide’s primary focus has been hematological malignancies, there is growing interest in its potential application in solid tumors. Research into its effectiveness in various solid tumor types is ongoing.
- Refining Safety Profiles: Efforts to minimize and manage side effects of lenalidomide continue, with a focus on developing more targeted interventions to enhance patient tolerability.
- Novel Targeted Protein Degradation Agents: The success of lenalidomide has spurred the development of novel agents designed for targeted protein degradation, offering new avenues for therapeutic exploration.
- Precision Medicine: The lessons learned from lenalidomide underscore the potential of personalized medicine. Tailoring treatment to an individual’s specific protein vulnerabilities holds promise for improving outcomes.
In conclusion, lenalidomide’s journey through clinical applications has provided invaluable lessons. It has demonstrated its efficacy and safety in treating hematological malignancies, illuminated the potential of targeted protein degradation as a cancer therapy strategy, and paved the way for exciting future directions and potential improvements in cancer care. The continued exploration of lenalidomide and its lessons learned will undoubtedly contribute to advancements in the field of oncology.