Liraglutide: A novel Treatment of Obesity
Abstract
liraglutide represents a significant advancement in the pharmacological treatment of obesity. With its ability to induce weight loss, improve metabolic parameters, and reduce cardiovascular risk factors, liraglutide can be a valuable part of a comprehensive obesity management program. However, its use must be balanced with careful monitoring for potential adverse effects, particularly those related to gastrointestinal and endocrine health. As obesity continues to be a global epidemic, treatments like liraglutide that offer a multifaceted approach to managing this complex condition are increasingly important in reducing the overall burden of obesity-related health issues.
Obesity is defined as a chronic, complex, and relapsing disease characterized by excessive adipose tissue accumulation that adversely impacts health. Historically perceived as a behavioral disorder influenced by overeating and insufficient physical activity, contemporary research now understands obesity as a multifaceted disease primarily driven by a breakdown in the homeostatic control of body weight, leading to uncontrolled fat accumulation. This paradigm shift views obesity not just as a lifestyle choice but as a serious health condition with significant metabolic, biomechanical, and psychosocial consequences.
The disease disrupts metabolic pathways and appetite signaling, which manifests in increased hunger and diminished feelings of fullness in affected individuals. These dysregulated biological processes contribute to a wide range of health complications. Obesity notably increases the risk of numerous conditions, including prediabetes, type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia, metabolic syndrome, cardiovascular disease, polycystic ovary syndrome (PCOS), nonalcoholic fatty liver disease (NAFLD), certain cancers like endometrial cancer, obstructive sleep apnea (OSA), depression, and osteoarthritis.
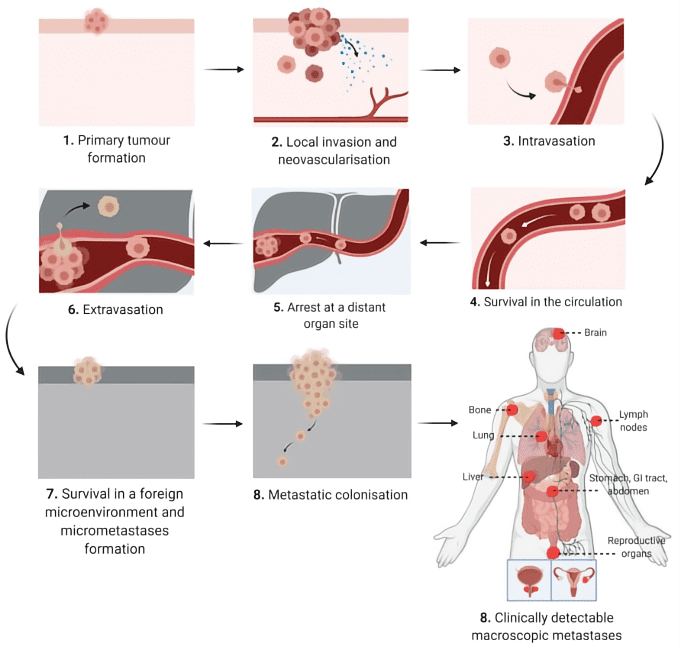
Figure 1. Schematic diagram depicting the keys steps of the metastatic cascade from initial presentation as an in situ tumor mass at the primary site to macroscopically detected metastatic lesions at secondary sites.
Moreover, obesity’s etiology is influenced by an array of factors including biological, genetic, epigenetic, environmental, neurological, and hormonal elements, all contributing to an imbalance between caloric intake and energy expenditure. This complex interplay underscores the multifactorial nature of obesity, complicating treatment strategies and requiring a multifaceted approach to effectively manage and potentially reverse its progression.
Mechanism of Action
The mechanism of action of Liraglutide, a glucagon-like peptide-1 (GLP-1) analog, in reducing food intake involves complex interactions within the body’s nervous system and gastrointestinal tract. Liraglutide is structurally similar to human GLP-1, sharing 97% of the amino acid sequence, which allows it to resist degradation by dipeptidyl peptidase-4 (DPP-4) and thus maintain a prolonged half-life of approximately 13 hours. This characteristic enables its once-daily dosing regimen, facilitating sustained therapeutic effects .
Central to the drug’s mechanism is its interaction with the GLP-1 receptors, which are dispersed throughout both the peripheral and central nervous systems, including key areas associated with metabolic control such as the pancreas, gastrointestinal tract, brain, and cardiovascular system. Upon binding to these receptors, Liraglutide influences the release of insulin and glucagon, leading directly to changes in blood glucose levels and indirectly affecting satiety and food intake .
Liraglutide’s action on the brain, particularly within regions controlling appetite and satiety, is crucial. GLP-1 receptors are found on nerve cells within the hypothalamus and brainstem areas like the nucleus tractus solitarius (NTS). Activation of these receptors modulates neuronal signals that reduce hunger and increase feelings of fullness. Studies in rodents and primates have shown that administering GLP-1 receptor agonists can lead to reduced food intake and body weight, highlighting the potential crossover of these effects to humans .
Moreover, the drug has been observed to slow gastric emptying, a physical process that contributes to prolonged satiety and reduced appetite. This effect is part of a broader influence on the gastrointestinal tract, which also includes modulation of gastric and pancreatic functions .
In addition to its effects on appetite regulation, Liraglutide engages in neuroendocrine signaling that reinforces its efficacy in obesity treatment. For instance, the drug’s impact on brain areas related to reward processing can diminish the perceived reward value of food, thereby curbing food-seeking behaviors and supporting sustained dietary changes.
This comprehensive approach to obesity treatment, combining metabolic, endocrine, and behavioral effects, positions Liraglutide as a significant therapeutic agent in the management of obesity, not just through its direct metabolic effects but also through its influences on eating behavior and satiety regulation .
Pharmacokinetic Properties of Liraglutide
Liraglutide’s unique pharmacokinetic profile is rooted in its innovative molecular design. Unlike native glucagon-like peptide-1 (GLP-1), which has a very short half-life due to rapid degradation by the enzyme dipeptidyl peptidase-4 (DPP-4), liraglutide is highly resistant to this enzyme. This resistance is due to a substitution at position 34 from lysine to arginine and the acylation of lysine at position 26 with a C16 fatty acid chain. This fatty acid chain enables liraglutide to bind to albumin in the bloodstream, reducing renal filtration and degradation, thereby extending its half-life to approximately 13 hours.
The pharmacokinetics of liraglutide allow for a once-daily subcutaneous administration, which is a significant advantage in promoting adherence to the treatment regimen among patients. It achieves peak plasma concentrations in 8 to 12 hours post-injection, and its steady-state concentration is reached after 2-3 days of dosing. The metabolism of liraglutide primarily involves its gradual absorption and proteolytic breakdown by endogenous enzymes, followed by metabolic degradation similar to that of large proteins without a specific organ as a major route of elimination.
Clinical studies have demonstrated that the pharmacokinetic properties of liraglutide are consistent across various patient groups, including those differentiated by gender, age, and body weight, indicating that dose adjustments based on these patient characteristics are generally not required. However, it should be noted that liraglutide’s pharmacokinetics can be altered in patients with renal impairment or severe gastrointestinal disease, where altered absorption or degradation might affect the drug’s efficacy and safety profile.
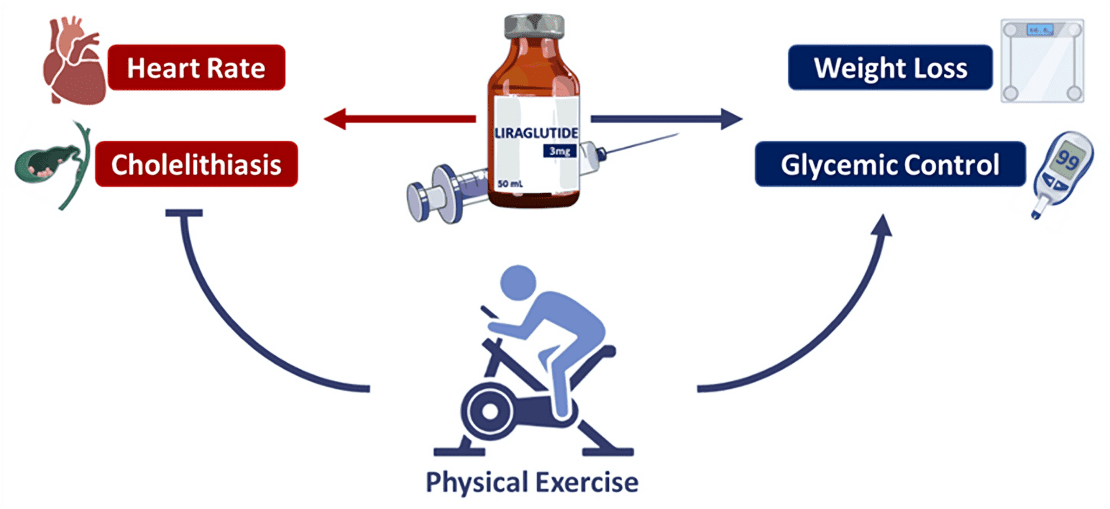
Figure 2. Summary of the effects of liraglutide associated with exercise or not
Clinical Impact and Efficacy
The clinical efficacy of liraglutide in the management of obesity has been robustly demonstrated through numerous randomized controlled trials. Liraglutide 3.0 mg, approved specifically for weight management, has been shown to achieve and sustain substantial weight loss over periods extending to 56 weeks and beyond. The SCALE (Satiety and Clinical Adiposity—Liraglutide Evidence) Obesity and Prediabetes trial, a landmark study, highlighted that participants taking liraglutide 3.0 mg lost significantly more weight—an average of 8% of their body weight—compared to those receiving the placebo.
Apart from weight loss, liraglutide has beneficial effects on various metabolic parameters, including improvements in glycemic control in type 2 diabetes patients, reductions in blood pressure, and lipid profile enhancements. These effects contribute to the reduction of overall cardiovascular risk, a critical factor in the clinical management of obesity. Furthermore, the drug’s efficacy in reducing prediabetic states to normal glycemia levels and in maintaining long-term weight loss offers a promising therapeutic avenue for a broad spectrum of patients with obesity-related metabolic dysfunctions.
Safety and Tolerability
While liraglutide offers significant benefits in the treatment of obesity, its safety and tolerability profile must be carefully considered. The most common adverse effects associated with liraglutide include gastrointestinal disturbances, such as nausea, vomiting, diarrhea, and constipation. These symptoms are generally transient and tend to diminish over time as the body adapts to the medication. Other potential side effects include a slight increase in heart rate and a risk of gallbladder disorders.
More serious concerns include the risk of pancreatitis, which necessitates immediate discontinuation of the medication if suspected, and the potential increase in thyroid C-cell tumors observed in rodent studies, although no causal relationship has been established in humans. As a result, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in those with Multiple Endocrine Neoplasia syndrome type 2.
Reference
- Alruwaili, H., Dehestani, B., & le Roux, C. W. (2021). Clinical impact of liraglutide as a treatment of obesity. Clinical pharmacology: advances and applications, 53-60.
- Annett, S., Moore, G., & Robson, T. (2020). Obesity and cancer metastasis: molecular and translational perspectives. Cancers, 12(12), 3798.
- Lin, C. H., Shao, L., Zhang, Y. M., Tu, Y. J., Zhang, Y., Tomlinson, B., … & Liu, Z. (2020). An evaluation of liraglutide including its efficacy and safety for the treatment of obesity. Expert opinion on pharmacotherapy, 21(3), 275-285.
- Xie, Z., Yang, S., Deng, W., Li, J., & Chen, J. (2022). Efficacy and safety of liraglutide and semaglutide on weight loss in people with obesity or overweight: A systematic review. Clinical epidemiology, 1463-1476.
- Barboza, J. J., Huaman, M. R., Melgar, B., Diaz-Arocutipa, C., Valenzuela-Rodriguez, G., & Hernandez, A. V. (2022). Efficacy of liraglutide in non-diabetic obese adults: a systematic review and meta-analysis of randomized controlled trials. Journal of Clinical Medicine, 11(11), 2998.
- Macêdo, A. P. A., Vieira, R. F. L., Brisque, G. D., Abud, G. F., & Pauli, J. R. (2022). Liraglutide and Exercise: A Possible Treatment for Obesity?. Obesities, 2(3), 285-291.




