Methylene Blue vs. COVID-19: How an Old Drug Blocks the Virus at the Cellular Doorway
Abstract
As the COVID-19 pandemic continues to challenge healthcare systems worldwide, the need for affordable, accessible, and effective antiviral therapies remains urgent. Recent research has spotlighted methylene blue, a well-established clinical dye, for its unexpected ability to inhibit SARS-CoV-2 entry into human cells. Specifically, methylene blue blocks the interaction between the viral spike (S) protein and the ACE2 receptor—a critical step in the infection process. This inhibition occurs at low micromolar concentrations, both in protein-binding assays and pseudovirus models, without the need for light activation. With its long history of clinical use, favorable pharmacokinetics, and global availability, methylene blue represents a promising candidate for repurposing as a COVID-19 therapeutic, particularly in resource-limited settings. Further clinical investigation is warranted to validate its antiviral potential and optimize its use in pandemic response strategies.
Introduction: Why We’re Still Searching for Effective Solutions
Even as the world gradually recovers from the peak of the COVID-19 pandemic, the search for safe, affordable, and accessible treatments continues. The virus that causes COVID-19—SARS-CoV-2—has proven to be highly transmissible and adaptable, with new variants constantly emerging. While vaccines and advanced antiviral drugs have significantly reduced the disease’s severity, not all populations have equal access to these medical advances. This gap has fueled interest in repurposing well-known, widely available drugs to fight the virus.
One such compound is methylene blue, a synthetic dye first used in medicine more than a century ago. Originally developed to treat malaria, methylene blue has been FDA-approved for conditions like methemoglobinemia and is included in the World Health Organization’s list of essential medicines. But could this old drug have new potential in combating COVID-19?
Recent research highlights the importance of the interaction between the virus’s spike protein and the human ACE2 receptor, the very first step in the virus’s ability to enter and infect cells. Blocking this protein-protein interaction (PPI) is a promising strategy to prevent infection. While many existing antivirals target later stages of the viral life cycle, stopping the virus at the “doorstep” could provide a powerful line of defense.
A study by Bojadzic et al. explores this very idea, showing that methylene blue can inhibit the spike–ACE2 interaction at low micromolar concentrations. If this mechanism holds true in human trials, methylene blue could offer a cost-effective and scalable solution, especially in regions where advanced COVID-19 therapies are scarce.
The idea of turning an age-old medicine into a modern antiviral is both scientifically exciting and socially impactful. It reflects a broader trend in global health: the smart repurposing of known compounds to meet urgent needs.
How the COVID-19 Virus Invades Our Cells: The Critical Role of Spike and ACE2
To understand how methylene blue might help in the fight against COVID-19, we need to take a closer look at how the virus actually enters human cells. The virus responsible for COVID-19—SARS-CoV-2—uses a highly efficient strategy to infect its host. The key lies in a specific interaction between two proteins: the virus’s spike (S) protein and the human angiotensin-converting enzyme 2 (ACE2) receptor.
The spike protein is the crown-like structure that gives coronaviruses their name. This protein enables the virus to bind to host cells, acting like a molecular “key” that fits into the “lock” of the ACE2 receptor. Once this connection is made, the virus can fuse with the host cell membrane and release its genetic material inside, starting the infection process.
This initial protein-protein interaction (PPI) between the spike protein and ACE2 is the first—and arguably most critical—step in viral entry. Disrupting this interaction could block the virus before it even enters the cell, effectively stopping infection at the source. This is why scientists and drug developers are actively searching for ways to interfere with this binding event.
While antibodies and vaccines often target the spike protein, they can be sensitive to viral mutations. Small-molecule inhibitors that block the spike–ACE2 interaction may offer a complementary approach—potentially one that’s less affected by variant-specific changes.
Methylene blue, as discussed in the recent research, shows promising activity in blocking the spike–ACE2 interaction, positioning itself as a potential antiviral agent. By interfering with this crucial molecular handshake, it may help prevent the virus from infecting host cells altogether.
Understanding the biology of viral entry not only highlights the value of early intervention strategies but also opens the door to repurposing existing drugs in creative and impactful ways.
Methylene Blue’s Hidden Superpower: Blocking the Virus Before It Enters
As the scientific community continues to explore innovative ways to fight COVID-19, a surprising candidate has emerged: methylene blue. While best known as a century-old dye with clinical applications in treating conditions like methemoglobinemia, recent research shows that methylene blue may have an unexpected and powerful antiviral function—it can block the interaction between the SARS-CoV-2 spike protein and the ACE2 receptor, the gateway through which the virus infects human cells.
A study published in Frontiers in Pharmacology revealed that methylene blue effectively inhibits the spike–ACE2 protein-protein interaction (PPI) in a concentration-dependent manner. Using a protein-based ELISA-style assay, researchers found that methylene blue showed an IC₅₀ (half-maximal inhibitory concentration) of around 3 μM, meaning it can significantly reduce binding between the spike protein and ACE2 at low micromolar levels.
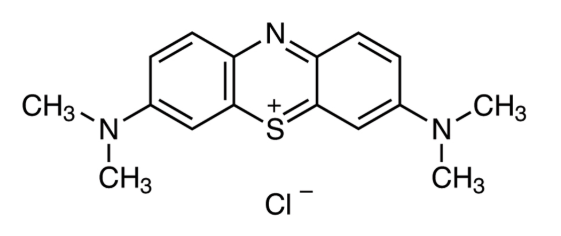
Figure 1. Methylene blue structure
But the story doesn’t end there. To validate these findings in a more biologically relevant model, the researchers tested methylene blue in a pseudovirus assay. They used human cells engineered to express ACE2 and exposed them to a pseudovirus bearing the SARS-CoV-2 spike protein. Once again, methylene blue demonstrated potent activity, preventing viral entry with an IC₅₀ of approximately 3.5 μM.
Unlike some antiviral treatments that require light activation (as with methylene blue’s historical use in pathogen inactivation), this inhibition occurred in the absence of light, suggesting that its antiviral activity in this context is driven purely by its molecular interaction with viral and cellular proteins.
What makes this finding even more promising is that such effective concentrations of methylene blue are achievable in humans through standard oral dosing. This raises the exciting possibility that methylene blue—widely available and inexpensive—could serve as a therapeutic option to help prevent SARS-CoV-2 infection, especially in regions with limited access to newer treatments.
From Malaria to Modern Medicine – Repurposing Methylene Blue for COVID-19
The idea of repurposing existing drugs has been a major theme in the global response to COVID-19. Among these, methylene blue stands out not just for its potential antiviral activity, but also for its rich medical history and established safety profile.
Methylene blue was first synthesized in the late 19th century and quickly became the first fully synthetic drug used in human medicine. Initially used to treat malaria, it later gained approval for conditions like methemoglobinemia, a blood disorder where oxygen delivery is impaired. Today, it’s listed on the WHO’s Model List of Essential Medicines due to its versatility and cost-effectiveness.
Unlike new antiviral drugs that often require years of testing and regulatory approval, methylene blue has already been widely studied in humans. Oral and intravenous forms are available, and pharmacokinetic studies show that plasma levels effective in vitro (around 3–4 µM) are readily achievable through standard dosing.
What makes methylene blue particularly attractive for COVID-19 is its affordability and accessibility, especially in low- and middle-income countries. These regions often lack access to cutting-edge antivirals or monoclonal antibody therapies, making an inexpensive oral antiviral a potential game changer.
However, methylene blue is not without precautions. It may cause side effects like nausea or vomiting at high doses and is contraindicated in individuals with G6PD deficiency or those taking certain antidepressants. Still, with careful use and proper dosing, its risk profile is manageable.
The drug’s polypharmacology—its ability to affect multiple biological pathways—may actually be an asset in COVID-19, where immune dysregulation, oxidative stress, and viral replication all contribute to disease severity.
Methylene blue may have started as a dye, but it’s showing serious promise as a modern antiviral weapon—especially in places that need it most.
What’s Next? Clinical Potential and Future Research
With promising lab results in hand, the next logical step for methylene blue as a potential COVID-19 treatment is clinical validation. While in vitro studies show it can block the SARS-CoV-2 spike protein from binding to ACE2 receptors, human trials are essential to confirm safety, dosing, and real-world efficacy.
A small Phase 1 clinical trial in Iran combined methylene blue with vitamin C and N-acetyl cysteine in critically ill COVID-19 patients. The majority showed rapid improvement, suggesting potential synergy with other antioxidant and anti-inflammatory agents. While this is encouraging, the sample size was small and lacked controls, highlighting the need for larger, randomized trials.
One intriguing aspect of methylene blue is its immune-modulating potential. In addition to antiviral activity, it may help balance immune responses—particularly beneficial in severe COVID-19 cases where cytokine storms can be deadly. There’s also evidence that it may restore T cell function by interfering with PD-1 signaling, which is often dysregulated during infection.
Another promising route is inhaled or nebulized delivery, which could directly target the lungs—the primary site of SARS-CoV-2 infection—while minimizing systemic exposure and side effects. This approach is already being explored in low-resource settings for respiratory conditions.
As new variants emerge and existing treatments face limitations, methylene blue offers hope not only as a cost-effective therapeutic, but also as a potential preventive measure. Its existing regulatory approval, oral availability, and wide global distribution make it an ideal candidate for repurposing—if further studies confirm its clinical utility.
In a world still battling COVID-19 and preparing for future pandemics, methylene blue is a reminder that sometimes, the oldest medicines can offer the newest solutions.
References
Bojadzic, D., Alcazar, O., & Buchwald, P. (2021). Methylene blue inhibits the SARS-CoV-2 spike–ACE2 protein-protein interaction–a mechanism that can contribute to its antiviral activity against COVID-19. Frontiers in Pharmacology, 11, 600372. https://doi.org/10.3389/fphar.2020.600372
Clifton, J., & Leikin, J. B. (2003). Methylene blue. American Journal of Therapeutics, 10(4), 289–291. https://doi.org/10.1097/00045391-200307000-00009
Schirmer, R. H., Adler, H., Pickhardt, M., & Mandelkow, E. (2011). Lest we forget you—methylene blue. Neurobiology of Aging, 32(12), 2325.e7–2325.e16. https://doi.org/10.1016/j.neurobiolaging.2010.12.012
Walter-Sack, I., et al. (2009). High absolute bioavailability of methylene blue given as an aqueous oral formulation. European Journal of Clinical Pharmacology, 65(2), 179–184. https://doi.org/10.1007/s00228-008-0563-x
Lan, J., Ge, J., Yu, J., Shan, S., Zhou, H., Fan, S., … & Wang, X. (2020). Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature, 581(7807), 215–220. https://doi.org/10.1038/s41586-020-2180-5
Shang, J., Wan, Y., Luo, C., Ye, G., Geng, Q., Auerbach, A., & Li, F. (2020). Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences, 117(21), 11727–11734. https://doi.org/10.1073/pnas.2003138117
Sivaraman, H., Er, S. Y., Choong, Y. K., Gavor, E., & Sivaraman, J. (2020). Structural basis of the SARS-CoV-2/SARS-CoV receptor binding and small-molecule blockers as potential therapeutics. Annual Review of Pharmacology and Toxicology, 61, 1–24. https://doi.org/10.1146/annurev-pharmtox-061220-093932
Tai, W., He, L., Zhang, X., Pu, J., Voronin, D., Jiang, S., … & Du, L. (2020). Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cellular & Molecular Immunology, 17(6), 613–620. https://doi.org/10.1038/s41423-020-0400-4
Cagno, V., Medaglia, C., Cerny, A., Cerny, T., Tapparel, C., & Cerny, E. (2020). Methylene blue has a potent antiviral activity against SARS-CoV-2 in the absence of UV-activation in vitro. bioRxiv. https://doi.org/10.1101/2020.08.14.251090
Gendrot, M., Andreani, J., Duflot, I., Boxberger, M., Mosnier, J., Le Bideau, M., et al. (2020). Methylene blue inhibits replication of SARS-CoV-2 in vitro. International Journal of Antimicrobial Agents, 56(6), 106202. https://doi.org/10.1016/j.ijantimicag.2020.106202
Alamdari, D. H., Moghaddam, A. B., Amini, S., Keramati, M. R., Zarmehri, A. M., Alamdari, A. H., et al. (2020). Application of methylene blue-vitamin C-N-acetyl cysteine for treatment of critically ill COVID-19 patients: report of a phase-I clinical trial. European Journal of Pharmacology, 885, 173494. https://doi.org/10.1016/j.ejphar.2020.173494
Fan, Z., Tian, Y., Chen, Z., Liu, L., Zhou, Q., He, J., et al. (2020). Blocking interaction between SHP2 and PD-1 denotes a novel opportunity for developing PD-1 inhibitors. EMBO Molecular Medicine, 12(9), e11571. https://doi.org/10.15252/emmm.201911571
Golwalkar, D. (2020). Treatment for COVID-19 using methylene blue. Medium. https://medium.com/@dr.deepak.golwalkar/treatment-for-covid-19-using-methylene-blue-d23fc5a31a4d
Vardhana, S. A., & Wolchok, J. D. (2020). The many faces of the anti-COVID immune response. Journal of Experimental Medicine, 217(6), e20200678. https://doi.org/10.1084/jem.20200678
Bistas, E., & Sanghavi, D. (2020). Methylene blue. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK541101/
Dicko, A., Roh, M. E., Diawara, H., Mahamar, A., Soumare, H. M., Lanke, K., et al. (2018). Efficacy and safety of primaquine and methylene blue for prevention of Plasmodium falciparum transmission in Mali: a phase 2, single-blind, randomised controlled trial. The Lancet Infectious Diseases, 18(6), 627–639. https://doi.org/10.1016/S1473-3099(18)30044-6