Microbiota-Derived AHR Ligands: Bridging Gut Health and Chronic Disease Therapy
Abstract
Emerging evidence highlights the critical role of microbiota-dependent metabolites and co-metabolites, acting as ligands for the aryl hydrocarbon receptor (AHR), in facilitating bidirectional communication between the host and microbiota, influencing various physiological processes. This communication is particularly prominent within the gastrointestinal tract. By binding to or activating AHR, these metabolites contribute to key physiological functions, especially in maintaining intestinal homeostasis. In recent studies, tryptophan metabolites have been screened to identify AHR ligands or activators with physiological relevance. The discovery of indole-based metabolites derived from microbiota as AHR ligands offers insight into how these compounds regulate interactions between gut microbiota and host intestinal homeostasis, as well as their implications for chronic gastrointestinal diseases and overall health. Understanding the mechanisms governing the production of these metabolites and their associated AHR activity could lead to effective treatments for inflammatory diseases and support human health. This review explores microbiota-derived AHR ligands from tryptophan and their role in modulating host-gut microbiota interactions, while also discussing potential therapeutic interventions for the future.
Introduction
The human gastrointestinal (GI) tract is a bioreactor with an incredibly diverse and dynamic microbial community referred to as the gut microbiota, which can be considered a functional organ. Over the past decade, tremendous strides have been taken toward understanding the composition and functional capacity of the gut microbiota that resides in the human GI tract1 . The mechanisms by which these microorganisms contribute to host health have been extensively investigated, and the gut microbiota has been recognized to play a critical role in human health by generating metabolites, which act both locally and systemically to influence human physiology and disease.2 Modern analytical methods have revealed that thousands of low molecular weight metabolites are produced that may mediate host-gut microbiota interactions.3 Many of these microbiota-dependent metabolites and co-metabolites have been demonstrated to correlate, either positively or negatively
with human health or disease. However, a causal mechanism of action has only been identified for a small fraction of the microbiota-dependent metabolites identified thus far. Currently, the most studied categories of metabolites involved in host-gut microbiota interactions, include short-chain fatty acids (SCFA), such as acetate, propionate, and butyrate, produced from the fermentation of dietary fiber, bile
acids secreted into the intestinal tract, and tryptophanderived microbial metabolites.4,5 The biological function of these classes of metabolites continues to be refined. Recent evidence has highlighted that many gut derived microbial metabolites modulate arylhydrocarbon receptor (AHR) activity, which facilitates
metabolic communication between the host and gut microbiota. The mechanisms underpinning such AHR-microbiota communication are multi-factorial, involving modulation of immune tolerance and response, intestinal homeostasis, carcinogenesis, and intestinal barrier integrity. AHR, a ligand-activated Among the most studied categories of metabolites involved in host-microbiota interactions are short-chain fatty acids (SCFAs), bile acids, and tryptophan-derived microbial metabolites. SCFAs such as acetate, propionate, and butyrate are produced through the fermentation of dietary fibers by the gut microbiota. These SCFAs are essential in modulating various physiological processes, including gut health, immune regulation, and metabolic functions. Similarly, bile acids secreted into the intestinal tract and microbial metabolites derived from tryptophan are gaining attention for their significant roles in human health. These metabolites contribute to gut homeostasis, immune response, and other critical biological functions.
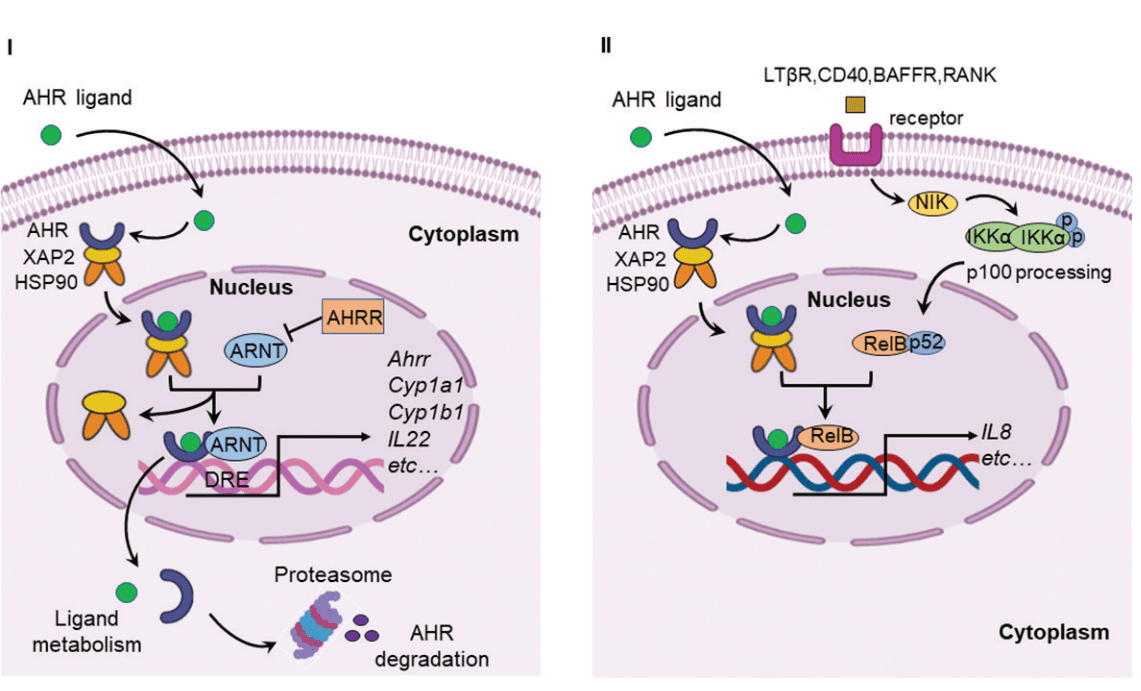
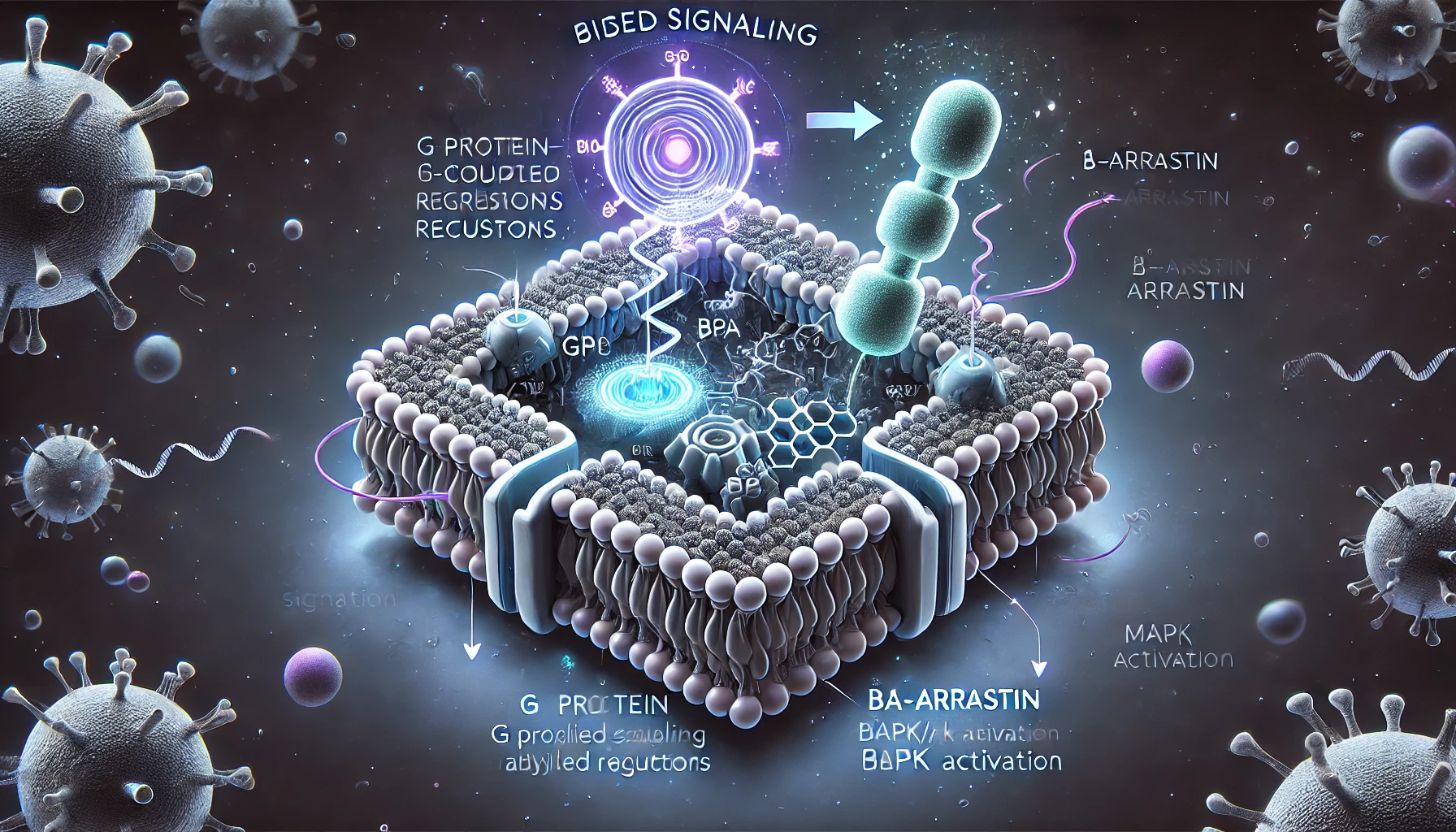
Figure 1. Canonical AHR signaling pathway and alternative AHR/RelB pathway.
Recent research has uncovered that many gut-derived microbial metabolites modulate the activity of the aryl hydrocarbon receptor (AHR), which serves as a key mediator of host-microbiota communication. The AHR, a ligand-activated transcription factor, plays a significant role in bridging microbial metabolism with the host’s immune system and other physiological processes. AHR is localized in the cytoplasm in an inactive state, associated with heat shock proteins (HSP90) and other molecules like X-associated protein 2 (XAP2) and P23. Upon binding to a ligand, AHR is activated and translocates into the nucleus, where it forms a functional DNA-binding transcription factor by dimerizing with the AHR nuclear translocator (ARNT) protein. This complex, once formed, regulates the expression of various genes involved in immune tolerance, intestinal integrity, and carcinogenesis, among other processes.
The interaction between AHR and the gut microbiota is multi-faceted, impacting several critical biological processes. AHR modulates immune responses and helps maintain intestinal homeostasis by influencing the production of immune cells and signaling molecules. In particular, AHR has been shown to interact with nuclear factor-kappa B (NF-κB), a transcription factor involved in the regulation of inflammatory responses. NF-κB is triggered by toll-like receptors (TLRs), which are activated by bacterial products such as lipopolysaccharides (LPS), leading to the expression of pro-inflammatory and cell survival genes. AHR agonists can suppress NF-κB-mediated gene expression, establishing a bidirectional relationship between these two important pathways. This interaction has been shown to be crucial for regulating inflammation and maintaining a balanced immune response in the gut.
Furthermore, AHR has been identified as a regulator of various chemokines and cytokines that are critical in immune response modulation. For instance, chemokines such as monocyte chemoattractant protein (MCP), interleukin 8 (IL-8), and CC-chemokine ligand 1 (CCL1) have been found to be regulated in an AHR-dependent manner. This regulation underscores the importance of AHR in orchestrating the immune response in the gut and beyond. Additionally, AHR, through its interaction with NF-κB, plays a role in controlling the expression of cytochrome P450 enzymes, specifically CYP1A1 and CYP1B1, which are important in xenobiotic metabolism.
Initially, AHR was studied primarily for its role as a sensor for environmental toxins, such as polycyclic aromatic hydrocarbons (PAHs) and halogenated aromatic hydrocarbons (HAHs), including the well-known toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Research into AHR originally focused on its role in mediating toxicity and tumorigenesis. However, more recent studies have shifted towards understanding its broader physiological role. AHR is now recognized for its immunomodulatory functions, particularly in relation to inflammatory diseases.
The evidence supporting AHR’s involvement in adaptive immune responses has continued to grow, linking its activity to conditions such as inflammatory bowel disease (IBD), multiple sclerosis (MS), rheumatoid arthritis (RA), cancer, and obesity. These findings suggest that AHR might play a role in the innate immune response to microbial invasion, particularly at barrier tissues like the gut lining. Activation of AHR by microbial metabolites, in combination with TLR ligands, could lead to an amplified inflammatory response, which has important implications for understanding chronic inflammatory diseases.
This growing body of research highlights the potential for AHR as a therapeutic target. Understanding how AHR regulates inflammation, particularly through interactions with microbiota-derived metabolites, could provide novel approaches for treating various chronic diseases. Further research is likely to reveal previously unknown biological processes that are regulated by AHR and will offer deeper insights into the complex relationship between the gut microbiota and host health. Researchers continue to explore AHR ligands, both exogenous and endogenous, with a focus on those produced by the gut microbiota, particularly metabolites derived from tryptophan and indole. By deepening our understanding of these mechanisms, we may unlock new therapeutic possibilities for managing inflammation and other microbiota-associated disorders.
AHR ligands and host metabolism in tryptophan catabolism
Several studies have identified the gut microbiota as a key driver in the degradation of tryptophan within the large intestine. Through microbial metabolic activity, various endogenous aryl hydrocarbon receptor (AHR) ligands are produced in the gastrointestinal (GI) tract, contributing to the regulation of gut health. For example, tryptamine (TrA), produced by Clostridium sporogenes and Ruminococcus gnavus through tryptophan decarboxylase activity, exhibits AHR-dependent anti-inflammatory effects in intestinal cells. TrA also serves as a precursor to indole-3-acetaldehyde (IAAld), another AHR ligand. Additionally, tryptophol (indole-3-ethanol, IEt), derived from IAAld by alcohol dehydrogenase, can regulate gut barrier function via AHR activation, along with other tryptophan metabolites like indole-3-pyruvic acid (IPyA) and IAAld.
Several gut bacteria, including Bacteroides fragilis, Bacteroides thetaiotaomicron, and species of Clostridium, can produce indole-3-acetic acid (I3A), another AHR ligand. I3A has been shown to regulate pro-inflammatory and oxidative effects in endothelial cells through AHR activation, promoting the expression of cyclooxygenase-2 (COX2). Elevated serum levels of I3A have been associated with increased mortality and cardiovascular events in chronic kidney disease (CKD) patients, suggesting its potential role as a biomarker for these conditions. Additionally, I3A was found to reduce cytokine-mediated lipogenesis in the liver, pointing to its protective role in lipid metabolism.
Strains of Lactobacillus reuteri and Lactobacillus johnsonii can generate indole-3-aldehyde (IAld) from tryptophan. In mice, AHR activation by IAld promotes the production of interleukin-22 (IL-22), which helps restore mucosal immune homeostasis. Another tryptophan metabolite, 3-methylindole (skatole), is produced by the decarboxylation of I3A by Lactobacillus species. Skatole can activate AHR in human intestinal and bronchial cells, although it acts as a weak AHR agonist and a competitive antagonist of TCDD-mediated AHR activation.
Tryptophan can also be metabolized into indole-3-lactic acid (ILA) through the actions of aromatic amino acid aminotransferase (ArAT) and indole-lactic acid dehydrogenase (ILDH). Bifidobacterium species and Clostridium sporogenes are known to produce ILA, which has been shown to suppress the transcription of the inflammatory cytokine IL-8 through AHR activation. Recent metabolomics studies identified that gut bacteria such as Peptostreptococcus russellii and Peptostreptococcus stomatis can produce indole-3-propionic acid (IPA) and indoleacetic acid (IAA), both of which have anti-inflammatory effects and are associated with gut health.
Indole metabolism is a rich source of AHR ligands. Indole is synthesized from tryptophan by microbial tryptophanase (TnaA), a process first identified in the late 19th century. Many bacterial species, including Escherichia coli, Bacteroides thetaiotaomicron, Clostridium tetani, and Bacillus alvei, can produce indole. This metabolite is present in the intestinal lumen and human feces at micromolar concentrations. Indole can be oxidized to 2-oxindole, which may contribute to the pathophysiology of hepatic encephalopathy. Both indole and 2-oxindole have been shown to activate AHR in the human GI tract.
Indoxyl sulfate (IS), another tryptophan-derived metabolite, is generated through the oxidation and sulfation of indole by hepatic enzymes such as cytochrome P450 2E1 (CYP2E1) and sulfotransferase 1A1 (SULT1A1). IS is typically eliminated via the kidneys but accumulates in high levels during kidney failure or CKD, where it acts as a uremic toxin. As a potent endogenous AHR agonist, IS selectively activates AHR in human hepatocytes and regulates the transcription of numerous genes. Studies have shown that the presence of gut flora is essential for IS production, linking gut microbiota with kidney disease. In CKD patients, serum concentrations of IS are significantly higher than in healthy individuals.
These findings suggest that the composition of the gut microbiota, the extent of bacterial metabolic activity, and the availability of tryptophan in the gut collectively determine the levels of microbiota-derived AHR ligands. However, further research is required to identify the optimal levels of these AHR ligands needed to maintain gut health and prevent disease. Understanding the balance between these factors may open new avenues for targeting the AHR pathway as a therapeutic strategy.
AHR role in obesity and gut/liver axis
Obesity is a global health issue exacerbated by Western diets that are high in calories, fats, and carbohydrates, and typically low in fruits and vegetables. Along with obesity, conditions such as fatty liver and increased visceral fat contribute to elevated inflammatory signaling. Emerging evidence points to the aryl hydrocarbon receptor (AHR) as playing a crucial role in the interaction between obesity and gut microbiota. Studies have shown that dietary supplementation in mice with an AHR ligand (e.g., FICZ) or with strains of Lactobacillus that produce AHR ligands can improve metabolic syndrome markers, including hepatic triglyceride levels, fasting glucose, and insulin levels in obese (ob/ob) mice.
In humans, a high body mass index (BMI) has been correlated with reduced levels of AHR-active tryptophan metabolites and overall AHR activation potential in fecal samples. Similarly, in fecal microbiota transplantation experiments, germ-free mice colonized with the microbiota of high-fat diet-fed mice exhibited reduced AHR activity compared to those colonized with microbiota from conventional diet-fed mice. This suggests that obesity-related changes in gut microbiota composition lead to a reduction in microbiota-derived AHR ligands. For example, obese mice have been shown to have a lower abundance of Bacteroidetes compared to lean mice, which may explain the reduced AHR activation.
One possible mechanism for these observations is that AHR activation in the gastrointestinal tract induces the production of interleukin-22 (IL-22), which has been shown to attenuate adverse metabolic parameters in obese mice. Studies have demonstrated that mice deficient in IL-22 receptor expression are more susceptible to metabolic dysfunction when fed a high-fat diet, suggesting that AHR activation plays a protective role against obesity by enhancing gut barrier function and supporting stem cell regulation in the intestinal epithelium.
However, studies examining AHR activation in the liver tell a different story. In transgenic mouse models with constitutively activated AHR in the liver, elevated CD36/fatty acid translocase expression results in a fatty liver phenotype due to increased fatty acid uptake. Additionally, the administration of a potent AHR ligand, 3-methylcholanthrene, has been shown to increase both liver triglyceride levels and CD36 expression, indicating that high-level AHR activation in the liver may contribute to fatty liver development.
Another mechanism involves the enzyme CYP1B1, which has been found to influence fatty acid metabolism and may prevent obesity. In mice lacking CYP1B1, there is a suppression of high-fat diet-induced obesity, attributed to decreased expression of stearoyl-CoA desaturase 1 (SCD1), the enzyme responsible for synthesizing monounsaturated fatty acids from saturated fatty acids. Additionally, the expression of genes regulated by peroxisome proliferator-activated receptor-alpha (PPARα) and PPAR-gamma (PPARγ), which are involved in fatty acid transport and adipocyte differentiation, are also reduced in these mice.
These findings suggest that AHR regulation of CYP1B1 expression may promote fatty acid synthesis, and that inhibiting CYP1B1 could serve as a potential therapeutic target for treating obesity. Indeed, studies have shown that dietary exposure to AHR antagonists, such as α-naphthoflavone, can inhibit fatty liver and obesity in mice fed a Western diet.
Therapeutic potential of AHR in clinical applications
Recent advancements in untargeted global metabolomics, which focus on identifying low molecular weight metabolites that affect the host, have enabled the screening of microbial metabolites that may activate the aryl hydrocarbon receptor (AHR) and influence gut microbiota homeostasis. These analyses, performed on cecal contents from conventional and germ-free mice, have identified 2,8-dihydroxyquinoline (2,8-DHQ) as a microbial-derived AHR ligand. There is growing evidence that AHR plays a critical role in regulating inflammatory signaling, affecting immune cells like Th17, Treg, and dendritic cells (DC). This progress in identifying microbiota-derived AHR ligands offers potential for developing therapeutic strategies for inflammatory diseases such as IBD, CNS disorders, and metabolic syndrome. Probiotics like Lactobacillus reuteri, which produce AHR agonists, have been used to alleviate inflammation, while direct administration of AHR agonists (e.g., indole and IPA) has been shown to reduce inflammation in colitis models. Moreover, diets enriched with tryptophan metabolites have demonstrated efficacy in limiting CNS inflammation. Targeted delivery of AHR agonists via nanoparticles is also being explored to minimize off-target effects, offering new approaches for protecting against infections and cancer.
Conclusions
Numerous studies have established the important role of the aryl hydrocarbon receptor (AHR) in maintaining gut homeostasis, both under normal conditions and in response to chemical or bacterial challenges. However, further research is needed to understand AHR’s role across different cell types within the intestinal tract, including immune cells in the gut epithelium, the underlying lamina propria, and the enteric nervous system. This will help determine the optimal levels of pseudo-endogenous ligands, such as tryptophan metabolites, or dietary ligands like indolo[3,2b]carbazole for maintaining gut health. The potential for these microbial or dietary AHR ligands to be used therapeutically also merits deeper investigation. For example, indole-3-carbinol has been shown to reduce the severity of Clostridium difficile infection in mice.
Interspecies differences in AHR activation also warrant attention, as there are structural differences between human and mouse AHRs. Notably, the AHR’s affinity for TCDD is ten times higher in mice than in humans, while indoxyl sulfate (IS) is a more potent ligand for the human AHR. Further exploration is needed to determine the precise function of each AHR ligand in various intestinal segments and cell types, as well as how microbial AHR ligands from tryptophan and indole metabolism vary between individuals. Comprehensive understanding of these mechanisms may pave the way for therapeutic strategies targeting the AHR.
Reference
- Dong, F., & Perdew, G. H. (2020). The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes, 12(1), 1859812.
- Bansal, T., Alaniz, R. C., Wood, T. K., & Jayaraman, A. (2010). The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proceedings of the National Academy of Sciences of the United States of America, 107(1), 228–233.
- Moyer, B. J., Rojas, I. Y., Kerley-Hamilton, J. S., Hazlett, H. F., Nemani, K. V., Trask, H. W., West, R. J., Lupien, L. E., Collins, A. J., Ringelberg, C. S., et al. (2016). Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity: Model for AHR activation by kynurenine via oxidized-LDL, TLR2/4, TGFβ, and IDO1. Toxicology and Applied Pharmacology, 300, 13–24.
- Vaidyanathan, B., Chaudhry, A., Yewdell, W. T., Angeletti, D., Yen, W.-F., Wheatley, A. K., Bradfield, C. A., McDermott, A. B., Yewdell, J. W., Rudensky, A. Y., et al. (2017). The aryl hydrocarbon receptor controls cell-fate decisions in B cells. Journal of Experimental Medicine, 214(1), 197–208.
- Marshall, N. B., Vorachek, W. R., Steppan, L. B., Mourich, D. V., & Kerkvliet, N. I. (2008). Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Journal of Immunology, 181(4), 2382–2391.
- Wikoff, W. R., Anfora, A. T., Liu, J., Schultz, P. G., Lesley, S. A., Peters, E. C., & Siuzdak, G. (2009). Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences of the United States of America, 106(10), 3698–3703.
- Riggio, O., Mannaioni, G., Ridola, L., Angeloni, S., Merli, M., Carla, V., Salvatori, F. M., & Moroni, F. (2010). Peripheral and splanchnic indole and oxindole levels in cirrhotic patients: A study on the pathophysiology of hepatic encephalopathy. The American Journal of Gastroenterology, 105(6), 1374–1381.