Neratinib: A Crucial Component of Targeted Treatment for CNS Metastases and HER2-Positive Breast Cancer
Abstract
In cases of advanced breast cancer (BC), there is a clear correlation between the expression of human epidermal growth factor receptor 2 (HER2) and an increased risk of metastasis, especially to the brain. Strong tyrosine kinase inhibitor neratinib exhibits notable anticancer effects by blocking the HER1, HER2, and HER4 signaling pathways. It has demonstrated effectiveness in reversing resistance in BC patients who had before received HER2-targeted therapy. For early-stage HER2-positive BC, seratinib is authorized for long-term adjuvant treatment. It also works well when combined with other medications for advanced HER2-positive BC, especially when it comes to minimizing central nervous system (CNS) metastases. Its effectiveness is supported by clinical evidence, and loperamide helps to mitigate the most frequent adverse effect, which is mild grade 3 diarrhea. For advanced BC patients, neratinib presents a viable approach to managing and averting brain metastases.
Introduction
About 20–30% of patients have HER2-positive breast cancer, which is an extremely aggressive subtype. In these patients, there is an increased risk of metastasis, particularly to the central nervous system (CNS), due to the overexpression of the human epidermal growth factor receptor 2 (HER2). Historically, aggressiveness has been associated with a poor prognosis and resistance to traditional treatments.
The development of targeted treatments, especially those that target HER2, has significantly enhanced the course of treatment for these individuals. Neratinib has been identified as a significant development among these treatments. Neratinib is an irreversible tyrosine kinase inhibitor that is taken orally. It blocks the signaling pathways that promote the growth of cancer by targeting several HER family receptors, such as HER1, HER2, and HER4.
The ability of neratinib to pass the blood-brain barrier, a major obstacle in the treatment of CNS metastases, is one of its greatest advantages. Neratinib’s tiny molecular structure allows it to overcome the brain’s barrier to entry, which is advantageous for both preventing and controlling central nervous system (CNS) involvement compared to many other conventional treatments that have difficulty doing so.
Patients with HER2-positive breast cancer now have a potent new therapy option in neratinib, especially those who are up against the difficult obstacle of CNS metastases. As further studies uncover its complete potential, neratinib is reaffirming its place as a key component of contemporary treatment approaches meant to enhance prognoses in this very aggressive cancer.
What is Neratinib?
An irreversible tyrosine kinase inhibitor (TKI) called neratinib is used to treat breast cancer that is HER2-positive. It primarily targets the HER1, HER2, and HER4 epidermal growth factor receptors, which are essential for the signaling cascades that promote the growth and survival of cancer cells. Neratinib, in contrast to reversible inhibitors, binds to these receptors permanently, guaranteeing sustained inhibition and successfully obstructing the downstream signaling cascades that promote tumor growth.
The ability of neratinib to pass the blood-brain barrier (BBB) is a major benefit as it often presents a hurdle when treating brain metastases linked to HER2-positive breast cancer. The brain-brain barrier (BBB) is a selective barrier that keeps many therapeutic medicines from reaching the central nervous system (CNS) while simultaneously protecting the brain. Because of their huge molecular size, conventional therapies like monoclonal antibodies like trastuzumab have difficulty reaching therapeutic levels in the brain. Neratinib, a small molecule TKI, can, however, cross the blood-brain barrier, making it an essential tool for treating CNS metastases in patients with HER2-positive breast cancer.
Neratinib is metabolized pharmacologically by the hepatic enzyme CYP3A4, with flavin-containing monooxygenase (FMO) catalyzing a secondary metabolism. Its half-life is 7 to 17 hours, and its primary mode of excretion is through feces. When consumed with high-fat foods, its absorption is greatly increased; nevertheless, because it is still sensitive to stomach pH, its bioavailability may be impacted.
Because of its distinct pharmacokinetic profile and capacity to block several HER receptors, celetinib is an essential part of HER2-targeted treatments, especially when it comes to treating CNS involvement.
Clinical Efficacy of Neratinib
Neratinib has undergone comprehensive clinical testing in numerous trials, proving to be beneficial in treating HER2-positive breast cancer, especially when combined with other treatments. Neratinib was proven to have strong anti-tumor effect in early clinical trials. Neratinib, for instance, had encouraging outcomes in patients with HER2-positive metastatic breast cancer (MBC) who had previously received trastuzumab in Phase 1 and 2 trials. When paired with chemotherapeutic drugs like paclitaxel and capecitabine, neratinib outperformed standard treatment in terms of objective remission rates (ORR) and progression-free survival (PFS).
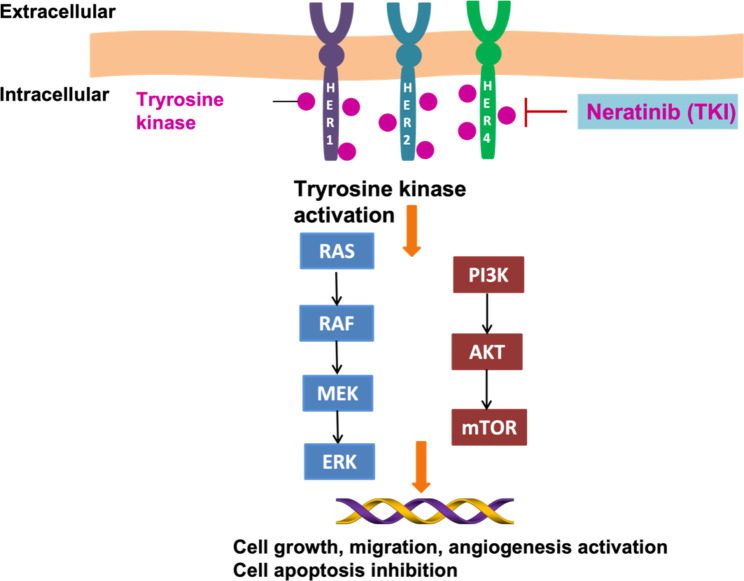
Fig. 1 Targeting of the HER1/2/4 receptor with the neratinib blocked breast cancer cell
Results of the phase 3 ExteNET trial, which assessed the effectiveness of neratinib as an extended adjuvant therapy, demonstrated a statistically significant improvement in patients’ invasive disease-free survival (iDFS) after they finished trastuzumab therapy for early-stage HER2-positive breast cancer. Neratinib was shown to lower the chance of recurrence in the trial, especially in hormone receptor-positive patients who began treatment within a year of finishing trastuzumab. Furthermore, the NEfERT-T trial demonstrated a decreased rate of central nervous system (CNS) metastases in patients receiving paclitaxel plus neratinib as opposed to patients receiving paclitaxel plus trastuzumab, underscoring neratinib’s potential to prevent brain metastases.
Another critical phase 3 trial, the NALA trial evaluated the effectiveness of capecitabine plus neratinib versus capecitabine plus lapatinib in patients with advanced HER2-positive breast cancer. According to the findings, there was a trend toward better overall survival (OS) and a significant improvement in PFS with the Neratinib combination. These results highlight the function of neratinib in extending survival and slowing the course of the disease in more advanced instances.
Role of Neratinib in Central Nervous System Metastases
Metastases to the central nervous system are a frequent and dangerous side effect of HER2-positive breast cancer that significantly impairs patient prognosis. The blood-brain barrier (BBB) makes it difficult for many therapeutic drugs to enter the body at high enough quantities, which makes therapy very difficult. Nonetheless, neratinib’s tiny molecular composition enables it to pass through the blood-brain barrier, providing special benefits in the management and avoidance of brain metastases.
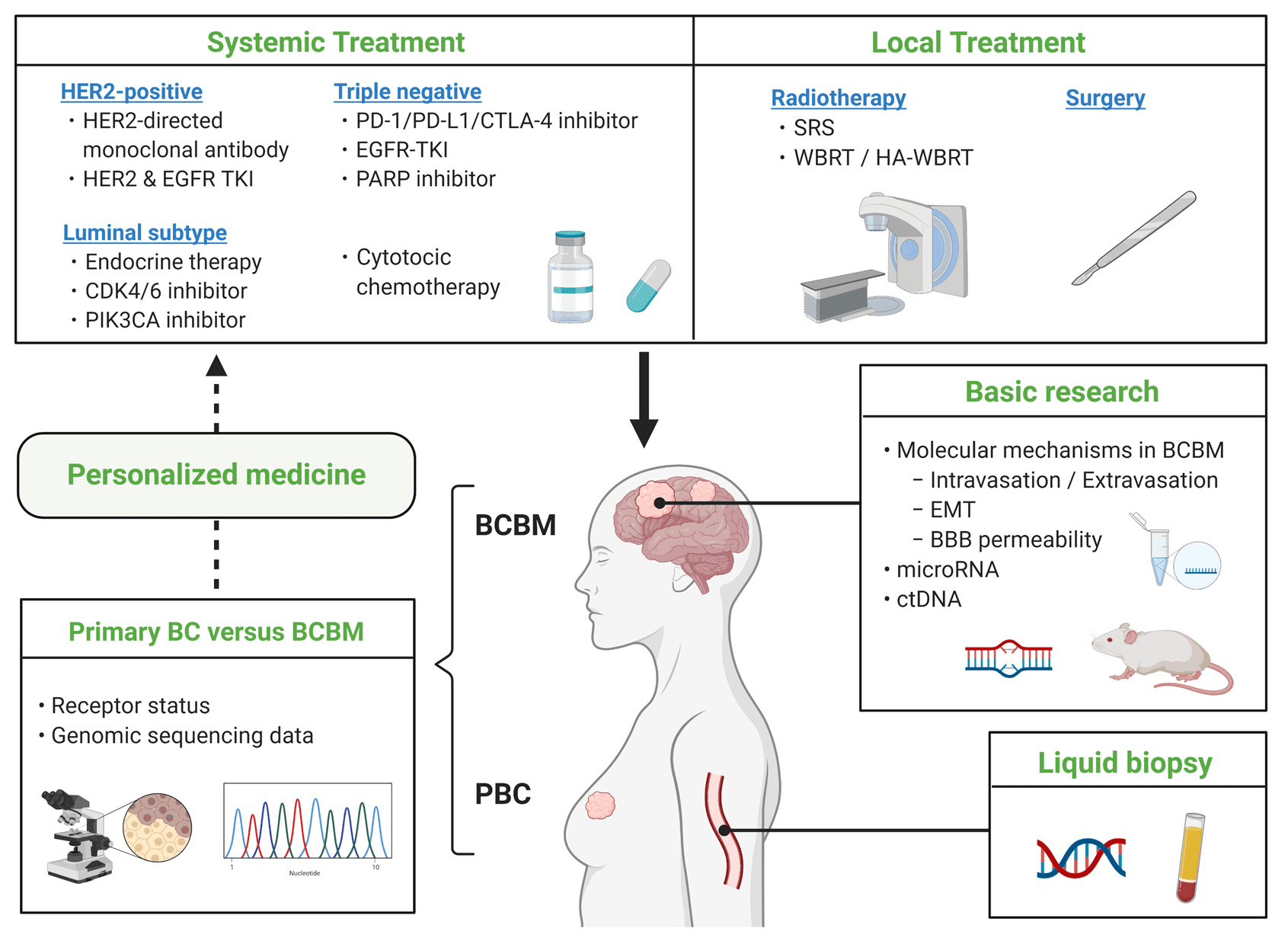
Fig.2 Breast Cancer Brain Metastasis—Overview of Disease State, Treatment Options and Future Perspectives
The CNS recurrence rate was considerably lower in the Neratinib-paclitaxel group compared to the trastuzumab-paclitaxel group in the NEfERT-T trial. According to this research, individuals with advanced HER2-positive breast cancer may benefit greatly from neratinib when used in conjunction with other treatments since it may be able to postpone or stop the development of CNS metastases. Furthermore, Neratinib dramatically decreased the size and progression of CNS tumors, particularly when paired with capecitabine, according to the TBCRC 022 study, which included patients with brain metastases.
These findings demonstrate the critical role that neratinib plays in treating one of the most difficult characteristics of HER2-positive breast cancer. According to Sakura et al. (2020), neratinib has the potential to improve both the prognosis and quality of life for patients suffering from CNS metastases in addition to improving survival rates.
Future directions and research
Even though neratinib has shown promising outcomes in the treatment of HER2-positive breast cancer, work is still being done to maximize its effectiveness and get past the obstacles presented by drug resistance. The goal of research is to comprehend the mechanisms underlying neratinib resistance, which continues to be a major obstacle to attaining long-term illness management. Combining Neratinib with other targeted or chemotherapeutic treatments is one example of how combination therapies are being investigated in order to avoid or overcome resistance.
The possibility of neratinib beyond HER2-positive breast cancer is a current research focus. Neratinib’s therapeutic spectrum may be expanded by treating tumors that are HER2-mutant or HER2-negative, according to preliminary research. Furthermore, continuing clinical trials are investigating the long-term results of patients treated with Neratinib, with an emphasis on overall survival and quality of life in particular. The purpose of these trials is to solidify the role of neratinib in the management of HER2-positive breast cancer as well as other possible cancer types.
Conclusion
Treatment for HER2-positive breast cancer has advanced significantly with the development of neratinib, especially for metastases to the central nervous system (CNS). It efficiently targets HER1, HER2, and HER4 as an irreversible tyrosine kinase inhibitor, resulting in long-term suppression of these important receptors. Because of its exceptional capacity to penetrate the blood-brain barrier, it is very useful in the management and avoidance of CNS metastases.
Neratinib has been shown to be effective in enhancing disease-free survival and lowering recurrence rates in clinical trials like ExteNET, NEfERT-T, and NALA, especially in patients who are hormone receptor-positive. Even if diarrhea is still a serious side effect, patients can still benefit from treatment because it can be controlled with the right prophylactic.
It is anticipated that ongoing research will further enhance patient outcomes by addressing medication resistance and broadening the use of neratinib beyond HER2-positive malignancies. Overall, neratinib has been a key player in the battle against HER2-positive breast cancer, offering patients a new hope, particularly those who are experiencing or are at risk of developing central nervous system metastases.




