New Progress of NASH Drug Therapy
Abstract
NSAH is one of the hot areas of drug development in recent years, accompanied by a high clinical failure rate. Therefore, the task of drug research and development in the field of non-alcoholic steatohepatitis is also imminent. This article mainly introduces some drugs that have entered clinical phase 3 and the latest research progress of DT-109.
Introduction
Nonalcoholic fatty liver disease refers to the presence of excess fat in the liver without the influence of alcohol. Excess fat will gradually invade the liver, resulting in less and less healthy tissue in the liver, and progress to nonalcoholic steatohepatitis, eventually causing cirrhosis and liver failure. Fatty liver and nonalcoholic steatohepatitis can be reversed and the liver can be restored to health, but once cirrhosis occurs, it cannot be reversed. This disease is the leading cause of chronic liver disease and the third most common cause of liver transplantation in the United States. Nonalcoholic fatty liver disease ( NAFLD ) is the most common chronic liver disease in the world, with a global prevalence of about 25 %, which brings huge economic and medical burden to society.
Nonalcoholic fatty liver disease ( NAFLD ) is the most common cause of abnormal levels of aminotransferases ( aminotransferases ) and has surpassed chronic hepatitis C infection as the most common cause of chronic liver disease and cirrhosis. Especially in the last two decades, its prevalence has been steadily rising, it is estimated that NAFLD has affected 30 % of the general population and 60-80 % of patients with type 2 diabetes.
The goal of NAFLD treatment :
( 1 ) As far as possible to reduce the risk of insulin resistance, impaired glucose tolerance, obesity, dyslipidemia, hypertension and other risk factors ;
( 2 ) As far as possible to reduce the accumulation of fat in the liver, to avoid the ‘ two hit ‘ on the liver damage ;
( 3 ) For patients who have progressed to NASH and liver fibrosis, cirrhosis, hepatocellular carcinoma ( HCC ) and its complications should be reduced.
In addition, mental factors, genetic susceptibility and intestinal flora can affect the occurrence and development of NAFLD. This article reviews the research progress of its treatment as follows.
For steatosis and NASH basic pathophysiological mechanisms, although some theories have been proposed, they have not been fully recognized. At present, it is generally believed that fat accumulation in the liver is the first cause of NASH, while pro-inflammatory cytokines, oxidative stress, iron accumulation, mitochondrial dysfunction, changes in the gut-liver axis, and secondary stimulation such as endomycin will further cause inflammation, cell damage and final fibrosis. According to the targets in the pathophysiology of NASH, the treatment options are divided into the following aspects : ( 1 ) systemic targets related to steatosis, glucose metabolism and adipogenesis ; ( 2 ) Targets related to immunomodulators ; ( 3 ) targets related to oxidative stress ; ( 4 ) objectives related to fibrosis ; ( 5 ) Targets related to apoptosis. At present, weight loss is recommended as a first-line treatment. However, weight loss is usually difficult to adhere to, so new therapeutic drugs are urgently needed.
There are 8 new NASH drugs in clinical phase III worldwide ( 2 have terminated phase III clinical trials ), more than 60 in clinical phase II ( including clinical phase I and II ), and 60 in clinical phase I. Currently, only India has approved the first and only NASH treatment drug – Saroglitazar.
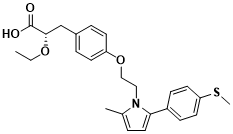
Figure 1 Structure of Saroglitazar
NASH, which has entered phase III clinical trials, is developing new drugs.
Lanifibranor(IVA377)
Lanifibranor is an oral small molecule preparation independently developed by Inventiva Pharma Company in the United States. It is a non-selective PPAR receptor agonist that can activate α, δ and γ receptors simultaneously. PPARβ / δ is highly expressed in the liver and is involved in lipid and carbohydrate metabolism, inflammatory response and other processes. Similar to PPARα, PPARδ can reduce the risk of cardiovascular disease, promote the production of hepatic glycogen and glucose utilization, reduce the expression of inflammatory factors and endoplasmic reticulum stress in the liver, reduce the activation of Kupffer cells and macrophages, and improve the inflammatory response in the liver. The results of phase IIb clinical trial ( NCT03008070 ) showed that Lanifibranor could simultaneously solve the abnormal glucose and lipid metabolism and inhibit the occurrence of inflammation and fibrosis due to its non-selective receptor. In this experiment, the main endpoint was the decrease of SAF activity score ≤ 2 points and no deterioration of CRN fibrosis score. The secondary endpoint was the histological improvement of NASH and serological indicators ( serum inflammatory markers, glucose metabolism and blood lipids ). Finally, only Lanifibranor 1200 mg treatment group reached the main endpoint ( 800 mg treatment group and placebo control group did not reach the endpoint ), confirming that Lanifibranor can delay or even reverse the histological progression of NASH to a certain extent. The ongoing phase III clinical trial ( NATiV3 ) aims to observe the efficacy of Lanifibranor in patients with NASH complicated with grade 2 / 3 liver fibrosis. The primary end point is the delay or reversal of NASH process and the improvement of liver fibrosis. The secondary end point is the improvement of liver NASH histological features, liver function indicators, and glucose and lipid metabolism serological indicators.

Figure 2 Structure of Lanifibranor
MSDC-0602K
MSDC-0602K is an insulin sensitizer developed by Cirius Therapeutics. It has similar pharmacological effects with the first-generation insulin sensitizer thiazolidinedione. Its mechanism of action is to specifically bind to the mitochondrial pyruvate carrier and regulate pyruvate into mitochondria. Finally, it can improve insulin resistance, glucose and lipid metabolism, reduce inflammation and improve serological indicators. Preclinical animal experiments have shown that MSDC-0602K can slow down the progression of NASH and reverse liver fibrosis by promoting lipid oxidative decomposition, reducing glycolipid synthesis, and inhibiting hepatic stellate cell activity. However, in the phase IIb clinical trial ( NCT02784444 ) , the MSDC-0602K treatment group did not reach the main clinical endpoint, that is, the improvement of NASH liver histology was not ideal. However, in patients with T2DM complicated with liver injury, liver injury and glucose metabolism were detected in a non-invasive manner, and MSDC-0602K showed its safety and potential efficacy. A phase III clinical trial ( NCT03970031 ) of MSDC-0602K for glycemic control and cardiovascular risk in NASH patients with T2DM is currently underway.
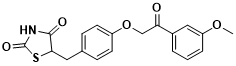
Figure 3 Structure of MSDC-0602K
Resmetirom(MGL-3196)
Resmetirom is an oral small molecule liver-targeted thyroid hormone receptor β ( THRβ ) agonist developed by Madrigal Pharmaceuticals. THRβ is the most expressed THR in the liver, which can promote cholesterol metabolism and reduce adipogenesis, thereby improving lipid abnormalities in NASH patients. Excessive thyroid hormones can not only specifically bind to THRβ in the liver, but also bind to THRα, causing adverse reactions in the heart and bone. Therefore, specific THRβ agonists can not only regulate lipid metabolism, but also avoid the side effects of thyroid hormones on heart and bone to a certain extent. Two phase II clinical trials on Resmetirom have confirmed that it can significantly reduce liver fat content in NASH patients and effectively reduce LDL and TG levels in the blood. Based on the above results, the ongoing phase III clinical trial ( NCT03900429 ) was designed to observe the efficacy of Resmetirom in the treatment of NASH patients with liver fibrosis for 52 weeks. The primary endpoint was that NASH was improved by biopsy and at least NAS score decreased by 2 points without deterioration of fibrosis. The secondary endpoint was a decrease in fibrosis grade by at least 1 grade without NASH deterioration and elevated LDL levels.
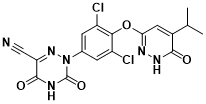
Figure 4 Structure of Resmetirom
Obeticholic Acid (OCA)
OCA is a FXR agonist developed by Intercept Pharmaceuticals Inc. FXR mainly regulates bile acid metabolism, participates in the synthesis and transport of bile acids, and has certain tissue specificity. In addition, FXR also plays an important role in regulating lipid metabolism and inhibiting liver inflammation. At the end of 2017, OCA was approved for the treatment of primary biliary cirrhosis. In 2019, the phase III clinical trial ( REGENERATE ) of OCA for NASH patients with grade 2 ~ 3 liver fibrosis achieved positive results in the mid-term ( 18th month ) analysis. The proportion of patients with at least grade 1 improvement in liver fibrosis and no deterioration of NASH reached significant statistical significance in the high-dose group ( 25 mg / d ). However, due to the unsatisfactory adverse reactions ( pruritus, hepatobiliary events, and elevated low-density lipoprotein cholesterol levels ) in the OCA treatment group, the FDA refused to approve OCA for the treatment of NASH.
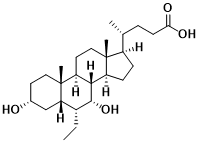
Figure 5 Structure of Obeticholic Acid
Elafibranor(GFT505)
Elafibranor and Lanifibranor developed by Genfit, France, are both PPAR receptor agonists, and their main targets are PPARα / δ. In 2011, Cariou et al.confirmed that Elafibranor can be used for the treatment of metabolic syndrome-related lipid and glucose metabolism abnormalities, and has a significant effect in reducing TG, fasting blood glucose, increasing HDL, and improving insulin resistance. Subsequently, in the phase II clinical trial of Elafibranor ( NCT01694849 ), compared with the 80 mg treatment group and the placebo control group, the proportion of patients with NASH remission and no progression of liver fibrosis in the 120 mg treatment group was higher. However, unfortunately, in May 2020, Elafibranor ‘s Phase III clinical trial ( RESOLVE-IT ) was declared to have ended in failure. Adult NASH patients with significant liver fibrosis were treated with Elafibranor 120 mg / d for 72 weeks and failed to achieve the primary endpoint of NASH remission and fibrosis without deterioration. At present, Elafibranor ‘s clinical trial for NASH indications has been terminated, and its phase III clinical trial for the treatment of primary biliary cirrhosis is ongoing.
Selonsertib(GS-4997)
Selonsertib is an oral apoptotic signal-regulated kinase 1 ( ASK1 ) inhibitor developed by Gilead in the United States. Hepatocyte tumor necrosis factor receptor-related factor 1 can promote hepatic steatosis by enhancing the activation of ASK1-mediated P38 / JNK signaling pathway. Inhibition of ASK1 can improve insulin resistance and steatosis . Phase II clinical trial ( NCT02466516 ) showed that Selonsertib 6 mg / d regimen could significantly alleviate liver fibrosis in patients with NASH and grade 2-3 liver fibrosis. However, phase III clinical trials ( STELLAR-4 and STELLAR-3 )were terminated due to missed primary endpoints and failure to reach the scheduled 48-week clinical endpoint, respectively, that is, 48-week Selonsertib monotherapy ( 18 mg / d and 6 mg / d ) had no anti-fibrosis effect in patients with NASH-induced bridging fibrosis or compensated cirrhosis. At present, Selonsertib ‘s clinical trial for NASH indications has been terminated, and its phase III clinical trial for diabetic nephropathy indications is ongoing.
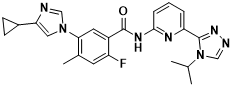
Figure 6 Structure of Selonsertib
Current research
On April 10,2023, ‘ DT-109 pregnants nonalcoholic steatohetitis in nonhuman primates ‘ was published online on Cell Metabolism impurities. This study reported that tripeptide DT-109 ( Gly-Gly-Leu ) dose-dependently attenuated steatohepatitis and fibrosis in mice. In order to improve the success rate of preclinical studies, this study developed a non-human primate animal model that mimics human NASH in histology and transcription.
Using a multi-omics approach combining transcriptomics, proteomics, metabolomics, and metagenomics, this study found that DT-109 not only stimulates fatty acid degradation and glutathione formation ( as found in mice ), but also reverses liver steatosis and prevents fibrosis progression in non-human primates by regulating microbial bile acid metabolism. The results described a highly transformative preclinical NASH model and emphasized the need for clinical evaluation of DT-109.
Worldwide, the prevalence of nonalcoholic fatty liver disease ( NAFLD ) and its more serious form, nonalcoholic steatohepatitis ( NASH ), is increasing at an alarming rate. Recently, the overall prevalence of NAFLD is estimated to be 32.4 %, while the prevalence of NASH in the general population is estimated to be 1.5 % -6.5 %. Although the prevalence of NAFLD is high, significant progress has been made in understanding the mechanisms leading to NASH, and great efforts have been made in drug development, there is currently no drug therapy to treat this disease.
Although many compounds are currently being evaluated for NASH, in clinical trials, many compounds either show no improvement or cause safety problems. A major obstacle to NASH drug development is the failure to translate preclinical findings into clinical settings. Mice are the most commonly used animals in preclinical studies of potential therapies for NASH. However, although a large number of NASH mouse models have been described, many models cannot accurately simulate human diseases and cannot be applied clinically. Therefore, the probability of successful clinical trials can be significantly improved by using robust preclinical models that are more easily converted into human NASH.
Although the current candidate drugs target the established pathways in the pathogenesis of NASH, the fact that no drug therapy has been approved highlights the necessity of identifying new targeted pathways. Recently, amino acid metabolic disorders are thought to be associated with NASH. In particular, recent studies by the research team and other researchers have found that impaired glycine metabolism is a pathogenic factor and therapeutic target for NASH and related cardiac metabolic diseases. Using NAFLD and NASH mouse models, the research team identified DT-109, a glycine-based tripeptide ( Gly-Gly-Leu ), to reduce steatohepatitis and liver fibrosis by inducing fatty acid ( FA ) degradation and antioxidant defense through nascent glutathione ( GSH ) biosynthesis.
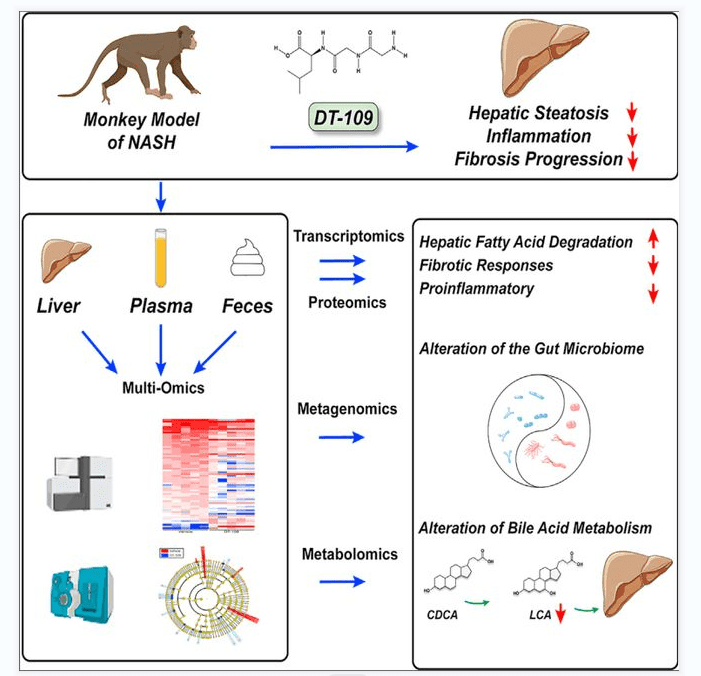
Figure 7[11] Graphical abstract
In order to improve the probability of success of DT-109 in clinical trials, this study evaluated the dose response of DT-109 and tested its efficacy and safety in non-human primates ( cynomolgus monkeys ) that mimic human diseases histologically and transcriptionally. This study further applied a multi-omics approach combining transcriptomics, proteomics, metabolomics, and metagenomics. The results revealed that DT-109 reversed liver steatosis and inhibited liver inflammation and fibrosis in non-human primates, not only by stimulating liver FA degradation and GSH formation, but also by regulating microbial bile acid ( BA ) metabolism.
Conclusion
Single-target drug therapy has little effect or considerable side effects, making it difficult for drugs to reach their main observation endpoints in clinical trials. In this case, the combined application of multi-target drugs for different pathogenesis of NASH may achieve better results. Based on the complex pathogenesis of NASH and the contradictory interaction of a single target in different signaling pathways, the development of a single drug for NASH in the future will cover multi-target or multi-drug and multi-target combined applications. In addition, although the clinical trials of new drugs for NASH are widely carried out worldwide, most of the experimental data are derived from the white population. The efficacy and safety of the experimental drugs in the Asian population remain to be discussed, which is also an important issue that must be paid attention to in the development of new drugs for NASH.
References
- Fraile JM, Palliyil S, Barelle C, Porter AJ, Kovaleva M. Non-Alcoholic Steatohepatitis (NASH) – A Review of a Crowded Clinical Landscape, Driven by a Complex Disease. Drug Des Devel Ther. 2021 Sep 22;15:3997-4009. doi: 10.2147/DDDT.S315724. PMID: 34588764; PMCID: PMC8473845.
- Welch RD, Billon C, Losby M, Bedia-Diaz G, Fang Y, Avdagic A, Elgendy B, Burris TP, Griffett K. Emerging Role of Nuclear Receptors for the Treatment of NAFLD and NASH. Metabolites. 2022 Mar 11;12(3):238. doi: 10.3390/metabo12030238. PMID: 35323681; PMCID: PMC8953348.
- Boeckmans J, Natale A, Rombaut M, Buyl K, Rogiers V, De Kock J, Vanhaecke T, M Rodrigues R. Anti-NASH Drug Development Hitches a Lift on PPAR Agonism. Cells. 2019 Dec 21;9(1):37. doi: 10.3390/cells9010037. PMID: 31877771; PMCID: PMC7016963.
- Cariou B. The metabolic triad of non-alcoholic fatty liver disease, visceral adiposity and type 2 diabetes: Implications for treatment. Diabetes Obes Metab. 2022 Feb;24 Suppl 2:15-27. doi: 10.1111/dom.14651. Epub 2022 Jan 24. PMID: 35014161.
- Tacke F, Weiskirchen R. Non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH)-related liver fibrosis: mechanisms, treatment and prevention. Ann Transl Med. 2021 Apr;9(8):729. doi: 10.21037/atm-20-4354. PMID: 33987427; PMCID: PMC8106094.
- Colca JR, McDonald WG, Adams WJ. MSDC-0602K, a metabolic modulator directed at the core pathology of non-alcoholic steatohepatitis. Expert Opin Investig Drugs. 2018 Jul;27(7):631-636. doi: 10.1080/13543784.2018.1494153. Epub 2018 Jul 17. PMID: 29950116.
- Colca J. NASH (nonalcoholic steatohepatitis), diabetes, and macrovascular disease: multiple chronic conditions and a potential treatment at the metabolic root. Expert Opin Investig Drugs. 2020 Feb;29(2):191-196. doi: 10.1080/13543784.2020.1715940. Epub 2020 Jan 23. PMID: 31928475.
- Zucchi R. Thyroid Hormone Analogues: An Update. Thyroid. 2020 Aug;30(8):1099-1105. doi: 10.1089/thy.2020.0071. Epub 2020 Apr 7. PMID: 32098589; PMCID: PMC7415871.
- Saponaro F, Sestito S, Runfola M, Rapposelli S, Chiellini G. Selective Thyroid Hormone Receptor-Beta (TRβ) Agonists: New Perspectives for the Treatment of Metabolic and Neurodegenerative Disorders. Front Med (Lausanne). 2020 Jul 9;7:331. doi: 10.3389/fmed.2020.00331. PMID: 32733906; PMCID: PMC7363807.
- Wirth EK, Puengel T, Spranger J, Tacke F. Thyroid hormones as a disease modifier and therapeutic target in nonalcoholic steatohepatitis. Expert Rev Endocrinol Metab. 2022 Sep;17(5):425-434. doi: 10.1080/17446651.2022.2110864. Epub 2022 Aug 11. PMID: 35957531.
- Qu P, Rom O, Li K, Jia L, Gao X, Liu Z, Ding S, Zhao M, Wang H, Chen S, Xiong X, Zhao Y, Xue C, Zhao Y, Chu C, Wen B, Finney AC, Zheng Z, Cao W, Zhao J, Bai L, Zhao S, Sun D, Zeng R, Lin J, Liu W, Zheng L, Zhang J, Liu E, Chen YE. DT-109 ameliorates nonalcoholic steatohepatitis in nonhuman primates. Cell Metab. 2023 Apr 4:S1550-4131(23)00091-8. doi: 10.1016/j.cmet.2023.03.013. Epub ahead of print. PMID: 37040763.
- Westerouen Van Meeteren MJ, Drenth JPH, Tjwa ETTL. Elafibranor: a potential drug for the treatment of nonalcoholic steatohepatitis (NASH). Expert Opin Investig Drugs. 2020 Feb;29(2):117-123. doi: 10.1080/13543784.2020.1668375. Epub 2019 Sep 25. PMID: 31523999.
- Wong VW. Current Prevention and Treatment Options for NAFLD. Adv Exp Med Biol. 2018;1061:149-157. doi: 10.1007/978-981-10-8684-7_12. PMID: 29956213.




